Category: Health Sciences and Medicine
ORIGINAL
Classifying alzheimer's disease from SMRI data using a hybrid deep learning approaches
Clasificación de la enfermedad de alzheimer a partir de datos SMRI utilizando un enfoque híbrido de aprendizaje profundo
Mathews Emmanuel1, J. Jabez2 *
1Department of Computer Science. Sathyabhama Institute of Science and Technology. Chennai, India.
2Department of Information Technology. Sathyabhama Institute of Science and Technology. Chennai, India.
Citar como: Mathews E, Jabez J. Classifying alzheimer’s disease from sMRI data using a hybrid deep learning approach. Salud, Ciencia y Tecnología - Serie de Conferencias 2024; 3:651. https://doi.org/10.56294/sctconf2024651
Submitted: 08-11-2023 Revised: 25-01-2024 Accepted: 22-03-2024 Published: 23-03-2024
Editor: Dr.
William Castillo-González ![]()
ABSTRACT
The chance of developing "Alzheimer's Disease (AD)" increases every 5 years after 65 years of age, making it a particularly common form of neurodegenerative disorder among the older population. The use of "Magnetic Resonance Imaging (MRI)" to diagnose AD has grown in popularity in recent years. A further benefit of MRI is that it provides excellent contrast and exquisite structural detail. As a result, some studies have used biological markers backed by "structural MRI (sMRI)" to separate AD populations, which indicate differences in brain tissue size and degradation of the nervous system. The lack of properly segmented regions and essential features by the existing models might affect classification accuracy for AD. The categorization of AD in this study is based on sMRI. In this research, the hybrid Deep-Learning Models "SegNet and ResNet (SegResNet)" have been proposed for segmentation, feature extraction, and to classify the AD. SegNet network is used to identify and segment specific brain areas. Edges and circles are the SegNet's first levels, whereas the deeper layers acquire more nuanced and useful features. SegNet's last deconvolution layer produces a wide range of segmented images linked to the 3 categorization labels "Cognitive Normal (CN)", "Mild Cognitive Impairment (MCI)", and "AD" which the machine has earlier found out. To increase classification performance, the attributes of each segmented sMRI image serve as strong features of the labels. To enhance the feature information used for classification, a feature vector is built by combining the values of the pixel intensity of the segmented sMRI images. ResNet-101 classifiers are then used for characterizing vectors to identify the presence or absence of AD or MCI in each sMRI image. In terms of detection and classification accuracy, the proposed SegResNet Model is superior to the existing KNN, EFKNN, AANFIS, and ACS approaches.
Keywords: Alzheimer's Disease; sMRI; Deep Learning; SegNet; ResNet.
RESUMEN
La probabilidad de desarrollar la "enfermedad de Alzheimer (EA)" aumenta cada 5 años a partir de los 65, lo que la convierte en una forma especialmente frecuente de trastorno neurodegenerativo entre la población de edad avanzada. En los últimos años se ha popularizado el uso de la resonancia magnética (RM) para diagnosticar la EA. Otra ventaja de la RM es que proporciona un contraste excelente y un detalle estructural exquisito. Como resultado, algunos estudios han utilizado marcadores biológicos respaldados por la "RM estructural (sMRI)" para separar poblaciones de EA, que indican diferencias en el tamaño del tejido cerebral y la degradación del sistema nervioso. La falta de regiones correctamente segmentadas y de características esenciales por parte de los modelos existentes podría afectar a la precisión de la clasificación de la EA. La categorización de la EA en este estudio se basa en sMRI. En esta investigación, se han propuesto los modelos híbridos de aprendizaje profundo "SegNet y ResNet (SegResNet)" para la segmentación, la extracción de características y la clasificación de la EA. La red SegNet se utiliza para identificar y segmentar áreas cerebrales específicas. Los bordes y los círculos son los primeros niveles de SegNet, mientras que las capas más profundas adquieren características más matizadas y útiles. La última capa de deconvolución de SegNet produce una amplia gama de imágenes segmentadas vinculadas a las 3 etiquetas de categorización "Cognitive Normal (CN)", "Mild Cognitive Impairment (MCI)" y "AD" que la máquina ha averiguado previamente. Para aumentar el rendimiento de la clasificación, los atributos de cada imagen sMRI segmentada sirven como características fuertes de las etiquetas. Para mejorar la información de características utilizada para la clasificación, se construye un vector de características combinando los valores de la intensidad de los píxeles de las imágenes sMRI segmentadas. A continuación, se utilizan clasificadores ResNet-101 para vectores de características con el fin de identificar la presencia o ausencia de EA o DCL en cada imagen sMRI. En términos de precisión de detección y clasificación, el modelo SegResNet propuesto es superior a los enfoques KNN, EFKNN, AANFIS y ACS existentes.
Palabras clave: Enfermedad de Alzheimer; sMRI; Deep Learning; SegNet; ResNet.
INTRODUCTION
After the age of 65, the number of persons afflicted by ADs is expected to increase every five years, according to the Alzheimer's Association. According to recent research, AD will impact one in every 85 individuals by the year 2050. The half-life recovery period for AD varies from three to ten years, depending on the patient's age at the time of diagnosis.(1)
AD patients who were diagnosed in their late 60s or early 70s had a median life span of between seven and ten years. Diagnosed AD patients' life expectancy is expected to fall by three years when they are in their 90s. Recently, thorough recommendations for diagnosing AD patients with MCI have been proposed, which might have a significant impact on early treatment and delay the onset of the disease.(2)
AD individuals with MCI are expected to have cognitive deterioration (memory lapses) without having a substantial effect on their daily lives. Two types of medical variations are common in people with MCI. There are two types of MCI patients: those that go from MCI to AD are referred to as MCI converters (mAD), and those who don't go from MCI to AD are referred to as stable MCI (aAD).(3)
In three years, 35 to 100 MCI patients may become dementia or AD patients, with an annual conversion rate of 5 to 10 percent. Individuals with mild amnesia and those with severe amnesia are both discriminated against. The more severe type of amnesia requires a lower normalized value on memory test results than the milder form, which requires a lower normalized value on amnesic test findings.(4)
There will be modest verbal memory impairment in those who have aAD in comparison to those with mAD, which is seen as an early commencement of the impairment, which is ideal for adjusting any treatment for the condition. Variations in biomarker abnormalities, clinical history, and health responses may all affect the two MCI subtypes. For people with MCI, there is no standard treatment method due to the wide range of medical examinations and underlying causes.(5)
As a result, the clinical history of MCI patients varies far from usual. However, several variables may lead to MCI, but not all of them can lead to the accompanying degeneration of the brain. To make a correct diagnosis, it is vital to recognize MCI patients with AD as the underlying cause and recognize them as such for the sake of making a diagnosis. The diagnostics must be completed as quickly as possible in the case of neurological degeneration. The classification of mAD and aAD may be used to solve this problem of qualitative diagnosis.(6)
"Alzheimer's Disease Assessment Scale Cognitive Subscale (AASCSC)", and the "Mini-Mental State Examination (MMSE)" show a widening disparity between estimates and current clinical results (ADAS-Cog). Predicting future clinical outcomes based on past data is essential to halting the advancement of the disease.(7,8,9,10,11,12,13)
AD individuals' cortical thickening patterns are complex, making it challenging to classify the condition. Maximize the number of samples used for training to cover all complicated patterns or choose relevant qualities that take into account variances between the two populations to overcome this issue. Neuronal-related problems may now be studied more thoroughly because of recent advances in brain scanning. This allows for more accurate and prompt treatment of AD.(8,14,15,16,17,18,19)
Problem Statement: The use of MRI to diagnose AD has grown in popularity in recent years. A further benefit of MRI is that it provides excellent contrast and exquisite structural detail. As a result, some studies have used biological markers backed by sMRI to separate AD populations, which indicate differences in brain tissue size and degradation of the nervous system. However the existing methods lack in classifying the MCI stages due to a lack of combining the feature extraction and classifications, also some methods fail in segmenting the proper region in the sMRI image.
Paper Contribution: The voxel properties of the area, length, and thickness are used in the studies. To be more specific, the whole dataset is randomly shuffled before being used for segmentation. In this research, the hybrid Deep-Learning Models SegResNet were proposed for segmentation, feature extraction, and to classify the AD. The sMRI training set has been used to generate segmented feature images using SegNet. Classifiers are trained using the SegNet segmented image's feature. The ResNet-101 classifier was trained without the usage of feature extraction techniques. According to SegNet + ResNet-101's performance on both controls and patients, the most important classification labels were CN, MCI, and AD. SegNet was first used to extract features from the ADNI dataset, and then ResNet-101 was used to classify three labels.
Paper Organization: Section 2 provides the recently published research on classifying AD, Section 3 will go through the methods and approaches that have been employed in both existing and proposed models, and Section 4 will cover the findings that it obtained by evaluating the three groups of modeling methods, and Section 5 will conclude up by looking ahead to potential future uses.
Related works
The researchers of (9) created a framework that combines "3D-Densely Connected Convolutional Networks (3D DenseNet)" and "Multi-task CNN" for learning features obtained from the segmented portion of the hippocampus. They were 88,9 % accurate in identifying AD and 76,2 % accurate in identifying MCI.
Comparable "3D-CNNs" were developed by the researchers of (10) using the "ResNet" framework and evaluated on several multi-class and binary operations. With the "Alzheimer's Disease Neuroimaging Initiative (ADNI)" dataset, they generated a cross-validation set for training and a limited set for testing. While these findings are encouraging, they are clouded by the fact that not any comparisons have been carried out to other conventional assessment frameworks, suggesting the model may be over-fit to the samples used for training. They were 89,1 % accurate in identifying AD and 79 % accurate in identifying MCI.
Dataset from the ADNI has been utilized to demonstrate by some researches which emphasizes on 3D-CNN application.(20,21,22,23,24,25,26,27) For obtaining features from MRIs and locating biomarkers for various AD sub-types, the "3D-CNN" was used. They were able to correctly identify AD in 90,9 % of cases and MCI in 80,3 % of cases.
After recognizing the shortcomings of traditional convolution, the researchers in (12) presented a new method called "Depth-wise Separable Convolution" to replace it. The cost of computing and variables had been drastically lowered since methods of "Transfer Learning" like "AlexNet" and "GoogLeNet" were utilized to train their concept. They were able to correctly identify AD in 91,5 % of cases and MCI in 81,5 % of cases.(28,29,30,31,32,33,34,35,36,37)
In some studies(13,38,39,40,41), the researchers suggest a "Deep Learning" inspired algorithm for "AD vs CN" categorization, wherein the deep characteristics of the "Left Hippocampal (LHM)" and "Right Hippocampal (RHM)" distinct volumes are extracted using a CNN-based volume estimating model. They were able to correctly identify AD in 92,4 % of cases and MCI in 82,6 % of cases.
METHODS
"Gray", "White", "Hippocampal Region Of Interest (ROI)", "Cerebrospinal Fluid", and "Cortical Thickness" are being investigated for segmentation as a foundation for feature extraction and measurement of the "Gyrification Index (GI)". A patient's whole brain is not taken into account when doing a clinical AD diagnosis that relies on feature extraction. Due to the lack of diagnostic support for several features, they might affect detection accuracy for AD. When using sMRI scans, the SegNet network is customized and trained individually to identify specific brain areas. Edges and circles are trained in the SegNet's first levels, whereas the deeper layers acquire more nuanced and useful properties. Seg-final Net's deconvolution layer generates a wide range of segmented images linked to the 3 categorization labels "CN", "MCI", and "AD" that were previously learned by machines. To increase classification performance, the attributes of each segmented sMRI image serve as strong features of the labels. A feature vector is constructed by merging the pixel intensity values of segmented to improve the feature data for classification. ResNet-101 classifiers eventually use the feature vector to identify the presence or absence of AD or MCI in each sMRI image. ResNet's outstanding performance in attribute-based categorization justifies its use here as a classifier. A schematic of the proposed SegResNet framework is shown in figure 1.
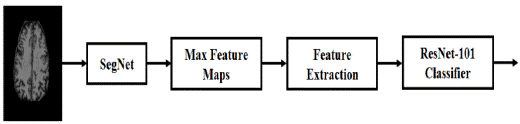
Figure 1. Proposed SegResNet Methodology
EFKNN
The distance estimates perform a vital part in the most common application of the approaches employed to the categorization: the identification of a link between a collection of entities. Contemporary pattern classification technologies such as the KNN technique are not reliant on image compressing and are therefore a viable alternative to its limitations. The Object-Membership processes used in standard KNN's grading process provide equal weight to all matched characteristics but lack several others. The EFKNN, which is based on the Fuzzy-Logic principle, exhibits not merely less variation but also more confidence when it classifies objects. The EFKNN also provides an advantageous vector between Object-Membership and Class-Membership. In (14,43,42) the EFKNN technique was proposed to find the most common way to represent a class by contrasting it against its "K-nearest neighbors". The EFKNN assigns "Fuzzy-Membership" with the sample and gives authorities discretionary decision-making power. To more accurately recognize AD's phases, this model was completely reviewed from the bottom up. This EFKNN technique was developed to categorize sMRI scans of the brain into cases of CN, MCI, and Pure AD. ADNI's MRI collection is utilized for training the models, which are subsequently put to the test. In this research, the database includes many people who have been diagnosed with CN, MCI, and AD.
AANFIS
In (15,44), an AANFIS approach was developed for AD classification. Neuronal morphology throughout biology is the primary focus of this research. The Neuro and Fuzzy systems are employed in a dual-pathway fashion. Initially, the fuzzy-interface blocks in a NN generate the MRI features. This NN could be modified to provide the desired results using the newly established model of Neuro-Fuzzy systems. In addition, FL takes advantage of the neuronal ability for training by rendering fuzzy networks more flexible to accommodate the execution of the objective. Fuzzy membership is employed as a decision-making framework for the sMRI image, and the NN has been utilized to improve its performance. There is evidence that the FL can convey the outcomes of expert analysis directly. In such a situation, it's an effort to regulate the use of certain terminology. The Member States' stance, which interprets some linguistic features numerically, requires an excessive amount of time to develop and govern. This is made feasible by combining the NN's automatic learning with the data. It improves the classifier's efficiency for predicting AD while decreasing training duration and costs.
ACS
An "Adaptive Clonal Selection (ACS)" technique was developed earlier to categorize sMRI scans into multi-class such as CN, MCI, and AD categories. The proposed ACS characterizes the essential features of the immunological response. This provides support for the hypothesis that the antigen can only mature inside the subset of cells that receive it, as opposed to the rest of the body. Comparable to evolutionary computation relying on mutations, this method excelled at focusing on the idea of clonal expansion and the development of affinity. The conception of evolutionary algorithms preferring the "fittest" is commonly used in this application. The ACS is capable of classifying the sMRI and transferring it to the next generations using the temporary information acquired through the cloning and affinity maturation method. This method primarily requires the generation of memory cells that are better able to catch antigens. This ACS technique introduces basic criteria from the concept of clonal expansion, that might assist in the creation of highly effective strategies for identifying template matches for the aforementioned "CN", "MCI", and "AD".
SegResNet
SegNet Architecture
SegNet consists of an "Encoder" and a "Decoder" architecture, which is then followed by a ResNet-based AD classification. Figure 2 shows the SegNet architecture. Objects are classified using the encoder's 13 convolution layers, which are similar to the first 13 convolution layers in VGG16's framed classification. As a result, trained weights are used to start the training process on bigger sets of data. Additionally, the encoder discards "Fully Connected Layers (FCN)" to preserve feature maps with higher resolution at the encoder's output. SegNet's encoder has been reduced from "134M" to "14.7M" in contrast to other recently established neural topologies to avoid an over-fitting problem. The decoder has the same number of layers as the encoder, thus there are 13 total. Softmax classification is used to calculate group probabilities from the decoder's final output.

Figure 2. SegNet Architecture
The SoftMax is the convolution procedure that generates feature maps for each encoder as shown in Figure 3. Then, "Batch Normalization" is implemented. Following this, a ReLU feature-wise corrected linear nonlinearity is applied. This is followed by a second 2x2 maximum pooling and non-overlapping stride 2 windows that are subsequently downsampled by a factor of 2.
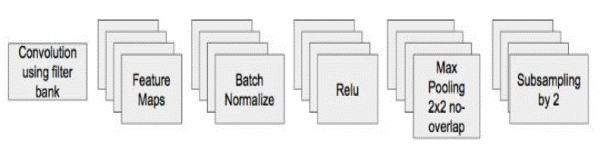
Figure 3. SegNet's Encoder Architecture
Segmentation Process by SegNet
An input sMRI image may be divided up into a plethora of smaller ones. By highlighting the image's edges, it makes it simpler to analyze (lines and curves in the image). The process of identifying each pixel such that pixels with the same label have the same attributes, such as color, texture, and intensity, is a basic process of segmentation. The attributes of pixels in certain areas of an image are the same, while the qualities of pixels in neighboring regions are dramatically different. It is necessary to terminate the segmentation procedure as soon as the ROI has been identified. Accordingly, the brain's anatomy must be correctly segmented to extract features from a specific area, since feature extraction relies on this precise segmentation. This may be used to view the brain's architecture, evaluate brain development, perform computerized processing and analysis of brain development, and plan brain surgery. Here SegNet takes sMRI images as input, it is necessary to establish shift invariability across tiny spatial translations by using a technique known as "Max-Pooling". In the case of a downsampled map, a vast number of possible contexts and spatial frames may be made available for each feature. To increase shift invariability and improve classification performance, adding additional layers of max-pooling and downsampling reduces the resolution of the feature map space. When edge definition is critical for segmentation, the rising loss on the image model is a hindrance. As a result, edge information in encoder feature maps must be acquired and stored before downsampling is performed. Encoder feature maps may be kept after downsampling when memory is not bound by interpretation.
Maximized pools are retained for each encoder feature map; this means that the positions at which a feature's maximum value is recorded for each pooling grid are preserved. It is possible to employ two bits for each window of 2x2 pooling, making it far more economical to store than a feature map in terms of floating-point accuracy. Because of the lower memory capacity, accuracy suffers only a little loss, but this is more than enough for most real-world applications. Max-pooling indices from the relevant feature map of the encoder are used by the decoder in the decoder architecture to upsample the input feature map.
Feature Extraction Process by SegNet:
To create a "sparse feature-map", the SegNet's upsampling is applied as shown in Figure 4. A decoder that is capable of learning dense feature maps is then able to apply convolution to the feature maps. As a further step, these maps are normalized in batches. Even though the encoder input image has RGB channels, several pipeline feature maps are created by the decoder according to an initial encoder that accepts the segmented sMRI image. A crucial difference is that feature maps produced by other elements of the system's encoder, the decoders have the ideal channel numbers and dimensions as the encoder's input. A tractable "Softmax Classifier" receives the higher-dimension feature scheme from the final decoder and applies it to its features. Each feature in this softmax has its category. The class with the greatest probability for each pixel in the segmented sMRI image under consideration is used to predict segmentation.
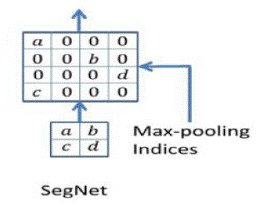
Figure 4. SegNet Upsampling
SegNet commonly consists of 4 encoders and 4 decoders. It is possible to use max-pooling and downsampling in this SegNet's encoder, and the corresponding decoder uses the gathered indices of max-pooling in upsampling as its input. Each convolution layer in the encoder and decoder is subjected to batch normalization. Non-linear ReLU is not used in the decoder to prevent biases after convolutions. All levels of the encoder and decoder employ the same 7 x 7 kernel to guarantee seamless labeling. This means that a feature in the 4th layer (that is, the deep feature map layer) may be tracked down to a backdrop window in the input picture that has 256 x 180 pixels. Using a basic SegNet, it is possible to examine and train several variations in a shorter amount of time.
Classification of AD by ResNet-101
sMRI images may be classified based on one or more features, and each feature belongs to a separate and exclusive class, which is the assumption of all classification methods. It is possible for an analyst to predetermine the classes or for them to be generated automatically (into groups of prototype classes where the analyst just provides the appropriate number of categories). The goals of classification and segmentation are intertwined since the former is another method of component labeling that may lead to the segmentation of multiple elements within an image. Visual classification examines the numerical qualities of several image features and categorizes the data. Two of the most typical phases of classification techniques are "Training" and "Testing". During the initial phase of training, distinguishing features of normal sMRI image information are used to provide a detailed characterization for every categorization category "Training class". During the testing phase, those feature-space partitions will be put into practice for categorizing aspects of sMRI images.
ResNet-101 Architecture
Each ResNet layer relies on mapping identity, which is essential. Adding a layer to an "Identity function f (x)=x" makes the new representation more efficient than the old one. Adding a second layer to this new representation may make it easier to reduce training mistakes since it is the best alternative for incorporating the training sMRI dataset. In addition, "f (x) = 0" is the simplest function that may be applied to a layer. Such concepts are complicated, yet they produced a surprisingly simple answer, a residual block, as a consequence of their consideration. This architecture had a huge impact on the development of deep neural networks. Figure 5 portrays the ResNet-101 architecture.
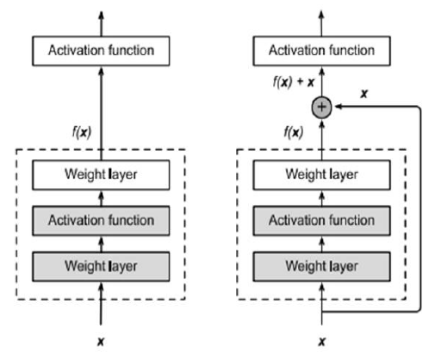
Figure 5. ResNet-101 Neural-Network Architecture
Figure 5 illustrates a portion of a neural network in ResNet-101 Architecture, where "x" represents the input which consists of the segmented sMRI image feature vector. The "Activation Function" is supposed to take as an input the "Ideal function f (x)" that the learner wishes to attain throughout the learning process. "Function (f)" should fit perfectly within the dashed box represented in the left image in Figure 5. When that layer is superfluous and the "input (x)" is required, it may be complicated. Figure 5 depicts a box with a dashed line that now only needs parameters to set up the deviation from identity since it yields "x + f. (x)". For optimization, mapping the residual is a straightforward task. As a result, all that's left is to get "f (x) to equal 0".
Figure 6 shows a ResNet Residual-Block on the right-hand side. VGG's entire three-layer convolution layer method is included in ResNet. There are 3 x 3 convolution layers in the residual block each with two output lines. Convolution layers now include batch normalization layers, as well as a ReLU activation function, built-in. As a result, these two convolutions are bypassed, and the input is simply summed before the final ReLU activation function. To sum the outputs of the two convolution layers, as shown in Figure 5, they must have the same size as the input. An additional 1 x 1 convolution layer must be added if the output size or stride is anticipated to vary, to accomplish an additional operation.
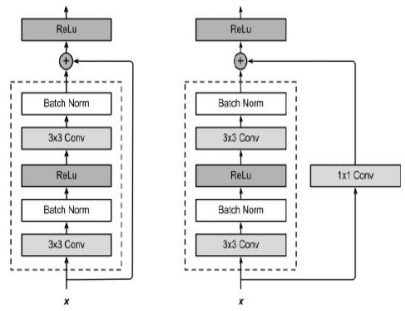
Figure 6. ResNet-101 Residual Block
Two strides are used when the shorting crosses across two-size feature maps. Every ResNet block has either two or three deep layers, depending on the size of the architecture (ResNet 18, 34, ResNet 50, 101, 152, etc.). This three-layer bottleneck block replaces every two-layer block in the 34-layer network, resulting in a 50-layer ResNet. In ResNets with 101 and 152 layers, more blocks of three layers are employed. Despite its greater depth, ResNet's 11,3 billion FLOPs and 152 layers have a lower level of complexity than the 15,3 or 19,6 billion FLOPs in VGG-16 or VGG-19 nets, respectively.
The input sMRI images are first and mainly scaled to 150*150 pixels to better separate features for classification. These sMRI images are then sent into SegNet, which is used for segmenting images to extract features from brain areas. Then the ResNet-100 is employed to get the desired outcomes in the real implementation of the suggested strategy. To train ResNet-101 using features extracted from SegNet, the sMRI dataset is ideal since each sMRI image already has a label. As a result, the ResNet-100 works with images using the same ADNI dataset, and the AD classifier is evaluated. sMRI images were classified into CN, MCI, and AD using the ADNI dataset's three labels.
RESULTS
Datasets: For conducting this study, unique sMRI images "Volumetric T1-weighted, Magnetization Prepared Rapid Gradient Echo (MPRAGE)" have been obtained using the open ADNI and KAGGLE datasets. A total of "2000 images of 210 (Male:105, Female:105)" with various categories "CN: 70, MCI 70, AD: 70" have been used.
Tools: Matlab, a computational programming environment, is used in an extensive range of diagnosing imaging systems. Matlab's user-friendlier interfaces make it a more rapid platform for incorporating new ideas. To evaluate all of the prototypes, we ran simulations with the Matlab software. We employed several methods, notably data generators, to enhance generalization, including "Rotating", "Intensity Modification", "Inversion", and more.
Performance Evaluation: The "Confusion Matrix (CM)" depicts an assortment of expected and actual categories, each of which is labeled as either "True Positive (TP)", "True Negative (TN)", "False Positive (FP)", or "False Negative (FN)". The CM reveals how effectively the model performs on the training data.
Accuracy: Accuracy in categorizing data frequently serves as a proxy for an application's overall quality. It's an important criterion used by experts to evaluate the classification system in its entirety. This means that the more accurate the categorization, the more efficiently the entire system will function. Using the CM, we'd been able to quickly quantify the progress that was made by using all these methods, as seen by Equation (1).
Accuracy=(TP+TN)/(TP+TN+FN+FP)*100 Eqà1
|
Table 1. Accuracy Comparison |
|||||
|
SUBJECTS |
KNN |
EFKNN |
AANFIS |
ACS |
SegResNet |
|
AD-sMRI1 |
81 |
92 |
94 |
96 |
98 |
|
AD-sMRI2 |
82 |
93 |
95 |
97 |
99 |
|
MCI-sMRI1 |
79 |
90 |
92 |
94 |
96 |
|
MCI-sMRI2 |
80 |
91 |
93 |
95 |
97 |
|
CN-sMRI1 |
85 |
96 |
98 |
99 |
99 |
|
CN-sMRI2 |
84 |
95 |
97 |
99 |
99 |
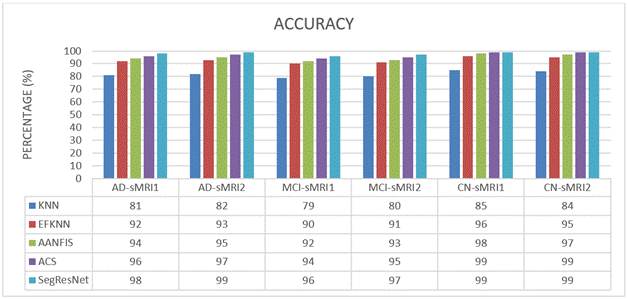
Figure 7. Accuracy Comparison
We compare and evaluate the accuracy efficacy of KNN, EFKNN, AANFIS, ACS, and SegResNet in recognizing CN, MCI, and AD using sMRI datasets during this research project. Table 1 and figure 7 demonstrate the outcomes of the different sMRI images' accuracy. In this case, a total of 6 images have been examined one after another. The suggested SegResNet method consistently outperforms the existing KNN, EFKNN, AANFIS, and ACS approaches. Finally, compared to the KNN, EFKNN, AANFIS, and ACS categorizations the SegResNet categorization has been proven to possess a greater accuracy in predicting MCI/AD patients.
Sensitivity / Recall:
In this part of the brain, we typically term the TP level, it was possible to correctly identify who would develop dementia. Similar to how individuals not having AD have been designated TN. Clinical investigation outcomes can additionally be correctly reflected by measures like the TP and TN. Each FN and FP have been reformulated in this novel setting. Findings from the research confirm the sensitivity's predictions about the TP's impact. According to the results of the sensitivity analysis, the method presented by Equation (2) can correctly identify the circumstances and calculate the fractions of FN and TP classes:
Sensitivity=TP/(TP+FN) Eqà2
|
Table 2. Sensitivity Comparison |
|||||
|
SUBJECTS |
KNN |
EFKNN |
AANFIS |
ACS |
SegResNet |
|
AD-sMRI1 |
77 |
88 |
91 |
94 |
97 |
|
AD-sMRI2 |
78 |
89 |
92 |
95 |
98 |
|
MCI-sMRI1 |
75 |
86 |
89 |
92 |
95 |
|
MCI-sMRI2 |
76 |
87 |
90 |
93 |
96 |
|
CN-sMRI1 |
81 |
92 |
95 |
98 |
99 |
|
CN-sMRI2 |
80 |
91 |
94 |
97 |
99 |
We compare and evaluate the sensitivity efficacy of KNN, EFKNN, AANFIS, ACS, and SegResNet in recognizing CN, MCI, and AD using sMRI datasets during this research project. Table 2 and Figure 8 demonstrate the outcomes of the different sMRI images' sensitivity. In this case, a total of 6 images have been examined one after another. The suggested SegResNet method consistently outperforms the existing KNN, EFKNN, AANFIS, and ACS approaches. Finally, compared to the KNN, EFKNN, AANFIS, and ACS categorizations the SegResNet categorization has been proven to possess a greater sensitivity in predicting MCI/AD patients.
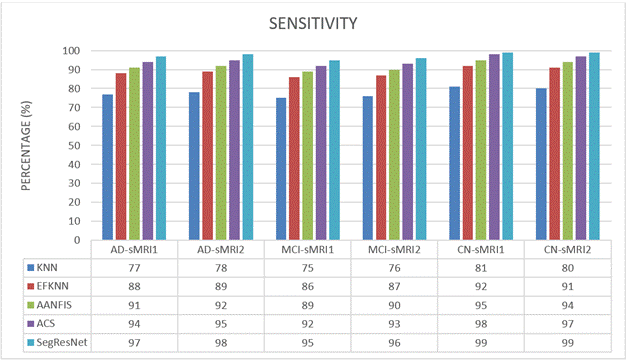
Figure 8. Sensitivity Comparison
CONCLUSION
The present research proposes a novel hybrid SegResNet architecture that outperforms state-of-the-art approaches in sMRI segmentation along with AD classification. To increase classification performance, the attributes of each segmented sMRI serve as strong features of the labels. A feature vector is constructed by merging the pixel intensity values of segmented images to improve the feature data for classification. ResNet-101 classifiers eventually use the feature vector to identify the presence or absence of AD or MCI in each sMRI image. To ensure that merely the most powerful and informative features spanning across all SegNet systems are supplied to the ResNet-100 classification algorithm, which averaged their maximal feature maps. According to SegResNet's performance on both controls and patients, the most important classification labels were CN, MCI, and AD. SegNet was first used to extract features from the ADNI dataset, and then ResNet-101 was used to classify three labels. The findings of these comparisons demonstrate that the proposed SegResNet approach has more classification accuracy and sensitivity than the state-of-the-art KNN, EFKNN, AANFIS, and ACS approaches. In the future, this research can be fine-tuned by introducing a post-processing process before classification to increase classification accuracy.
REFERENCES
1. Abrol, M. Bhattarai, A. Fedorov, Y. Du, S. Plis, and V. Calhoun, ‘‘Deep residual learning for neuroimaging: An application to predict progression to Alzheimer’s disease,’’ J. Neurosci. Methods, vol. 339, 2020, Art. no. 108701.
2. Basher, B. C. Kim, K. H. Lee, and H. Y. Jung, ‘‘Volumetric feature-based Alzheimer’s disease diagnosis from sMRI data using a convolutional neural network and a deep neural network,’’ IEEE Access, vol. 9, pp. 29870–29882, 2021.
3. Amado DPA, Diaz FAC, Pantoja R del PC, Sanchez LMB. Benefits of Artificial Intelligence and its Innovation in Organizations. AG Multidisciplinar 2023;1:15-15. https://doi.org/10.62486/agmu202315.
4. Batista-Mariño Y, Gutiérrez-Cristo HG, Díaz-Vidal M, Peña-Marrero Y, Mulet-Labrada S, Díaz LE-R. Behavior of stomatological emergencies of dental origin. Mario Pozo Ochoa Stomatology Clinic. 2022-2023. AG Odontologia 2023;1:6-6. https://doi.org/10.62486/agodonto20236.
5. Bhatele KR, Bhadauria SS (2020) Brain structural disorders detection and classification approaches: a review. Artif Intell Rev 53(5):3349–3401.
6. Caero L, Libertelli J. Relationship between Vigorexia, steroid use, and recreational bodybuilding practice and the effects of the closure of training centers due to the Covid-19 pandemic in young people in Argentina. AG Salud 2023; 1:18. https://doi.org/10.62486/agsalud202318.
7. Cavalcante L de FB. Feminicide from the perspective of the cultural mediation of information. Advanced Notes in Information Science 2023;5:24-48. https://doi.org/10.47909/978-9916-9906-9-8.72.
8. Chalan SAL, Hinojosa BLA, Claudio BAM, Mendoza OAV. Quality of service and customer satisfaction in the beauty industry in the district of Los Olivos. SCT Proceedings in Interdisciplinary Insights and Innovations 2023;1:5-5. https://doi.org/10.56294/piii20235.
9. Chávez JJB, Trujillo REO, Hinojosa BLA, Claudio BAM, Mendoza OAV. Influencer marketing and the buying decision of generation «Z» consumers in beauty and personal care companies. SCT Proceedings in Interdisciplinary Insights and Innovations 2023;1:7-7. https://doi.org/10.56294/piii20237.
10. D. P. Veitch, M. W. Weiner, P. S. Aisen, L. A. Beckett, N. J. Cairns, R. C. Green, D. Harvey, C. R. Jack, W. Jagust, J. C. Morris, R. C. Petersen, A. J. Saykin, L. M. Shaw, A.W. Toga, and J. Q. Trojanowski, ``Understanding disease progression and improving Alzheimer's disease clinical trials: Recent highlights from the Alzheimer's disease neuroimaging initiative,'' Alzheimer's Dementia, vol. 15, no. 1, pp. 106-152, 2019.
11. Diaz DPM. Staff turnover in companies. AG Managment 2023;1:16-16. https://doi.org/10.62486/agma202316.
12. Espinosa JCG, Sánchez LML, Pereira MAF. Benefits of Artificial Intelligence in human talent management. AG Multidisciplinar 2023;1:14-14. https://doi.org/10.62486/agmu202314.
13. Figueredo-Rigores A, Blanco-Romero L, Llevat-Romero D. Systemic view of periodontal diseases. AG Odontologia 2023;1:14-14. https://doi.org/10.62486/agodonto202314.
14. G. Folego, M. Weiler, R. F. Casseb, R. Pires, and A. Rocha, ‘‘Alzheimer’s disease detection through whole-brain 3D-CNN MRI,’’ Frontiers Bioeng.Biotechnol., vol. 8, p. 1193, Oct. 2020.
15. Gonzalez-Argote J, Castillo-González W. Productivity and Impact of the Scientific Production on Human-Computer Interaction in Scopus from 2018 to 2022. AG Multidisciplinar 2023;1:10-10. https://doi.org/10.62486/agmu202310.
16. Hernández-Flórez N. Breaking stereotypes: “a philosophical reflection on women criminals from a gender perspective". AG Salud 2023;1:17-17. https://doi.org/10.62486/agsalud202317.
17. Hinojosa BLA, Mendoza OAV. Perceptions on the use of Digital Marketing of the micro-entrepreneurs of the textile sector of the Blue Gallery in the emporium of Gamarra. SCT Proceedings in Interdisciplinary Insights and Innovations 2023;1:9-9. https://doi.org/10.56294/piii20239.
18. J. Liu, M. Li, Y. Luo, S. Yang, W. Li, and Y. Bi, ‘‘Alzheimer’s disease detection using depthwise separable convolutional neural networks,’’ Comput. Methods Programs Biomed., vol. 203, May 2021, Art. no. 106032.
19. J. Zhang, B. Zheng, A. Gao, X. Feng, D. Liang, and X. Long, ``A 3D densely connected convolution neural network with connection-wise attention mechanism for Alzheimer's disease classification,'' Magn. Reson. Imag., vol. 78, pp. 119-126, May 2021.
20. Lamorú-Pardo AM, Álvarez-Romero Y, Rubio-Díaz D, González-Alvarez A, Pérez-Roque L, Vargas-Labrada LS. Dental caries, nutritional status and oral hygiene in schoolchildren, La Demajagua, 2022. AG Odontologia 2023;1:8-8. https://doi.org/10.62486/agodonto20238.
21. Ledesma-Céspedes N, Leyva-Samue L, Barrios-Ledesma L. Use of radiographs in endodontic treatments in pregnant women. AG Odontologia 2023;1:3-3. https://doi.org/10.62486/agodonto20233.
22. Lee, B.; Yamanakkanavar, N.; Choi, J.Y. Automatic segmentation of brain MRI using a novel patch-wise U-net deep architecture. PLoS ONE 2020, 15, e0236493.
23. Lopez ACA. Contributions of John Calvin to education. A systematic review. AG Multidisciplinar 2023;1:11-11. https://doi.org/10.62486/agmu202311.
24. M. A. DeTure and D. W. Dickson, ``The neuropathological diagnosis of Alzheimer's disease,'' Mol. Neurodegeneration, vol. 14, no. 1, pp. 1-18, Aug. 2019.
25. M. Emmanuel and J. Jabez, "An Advanced Adaptive Neuro-Fuzzy Inference System for Classifying Alzheimer's Disease Stages From SMRI Images," 2023 Advanced Computing and Communication Technologies for High-Performance Applications (ACCTHPA), Ernakulam, India, 2023, pp. 1-8, doi: 10.1109/ACCTHPA57160.2023.10083347.
26. M. Liu, F. Li, H. Yan, K. Wang, Y. Ma, L. Shen, and M. Xu, ‘‘A multi-model deep convolutional neural network for automatic hippocampus segmentation and classification in Alzheimer’s disease,’’ NeuroImage, vol. 208, Mar. 2020, Art. no. 116459.
27. Marcillí MI, Fernández AP, Marsillí YI, Drullet DI, Isalgué RF. Older adult victims of violence. Satisfaction with health services in primary care. SCT Proceedings in Interdisciplinary Insights and Innovations 2023;1:12-12. https://doi.org/10.56294/piii202312.
28. Marcillí MI, Fernández AP, Marsillí YI, Drullet DI, Isalgué VMF. Characterization of legal drug use in older adult caregivers who are victims of violence. SCT Proceedings in Interdisciplinary Insights and Innovations 2023;1:13-13. https://doi.org/10.56294/piii202313.
29. Mathews Emmanuel, & Jabez, J. (2022). An Enhanced Fuzzy Based KNN Classification Method for Alzheimer's Disease Identification from SMRI Images. JOURNAL OF ALGEBRAIC STATISTICS, 13(3), 89–103.
30. Moraes IB. Critical Analysis of Health Indicators in Primary Health Care: A Brazilian Perspective. AG Salud 2023;1:28-28. https://doi.org/10.62486/agsalud202328.
31. Ogolodom MP, Ochong AD, Egop EB, Jeremiah CU, Madume AK, Nyenke CU, et al. Knowledge and perception of healthcare workers towards the adoption of artificial intelligence in healthcare service delivery in Nigeria. AG Salud 2023;1:16-16. https://doi.org/10.62486/agsalud202316.
32. Peñaloza JEG, Bermúdez L marcela A, Calderón YMA. Perception of representativeness of the Assembly of Huila 2020-2023. AG Multidisciplinar 2023;1:13-13. https://doi.org/10.62486/agmu202313.
33. Pérez DQ, Palomo IQ, Santana YL, Rodríguez AC, Piñera YP. Predictive value of the neutrophil-lymphocyte index as a predictor of severity and death in patients treated for COVID-19. SCT Proceedings in Interdisciplinary Insights and Innovations 2023;1:14-14. https://doi.org/10.56294/piii202314.
34. Prado JMK do, Sena PMB. Information science based on FEBAB’s census of Brazilian library science: postgraduate data. Advanced Notes in Information Science 2023;5:1-23. https://doi.org/10.47909/978-9916-9906-9-8.73.
35. Pupo-Martínez Y, Dalmau-Ramírez E, Meriño-Collazo L, Céspedes-Proenza I, Cruz-Sánchez A, Blanco-Romero L. Occlusal changes in primary dentition after treatment of dental interferences. AG Odontologia 2023;1:10-10. https://doi.org/10.62486/agodonto202310.
36. Quiroz FJR, Oncoy AWE. Resilience and life satisfaction in migrant university students residing in Lima. AG Salud 2023;1:9-9. https://doi.org/10.62486/agsalud20239.
37. Roa BAV, Ortiz MAC, Cano CAG. Analysis of the simple tax regime in Colombia, case of night traders in the city of Florencia, Caquetá. AG Managment 2023;1:14-14. https://doi.org/10.62486/agma202314.
38. Rodríguez AL. Analysis of associative entrepreneurship as a territorial strategy in the municipality of Mesetas, Meta. AG Managment 2023;1:15-15. https://doi.org/10.62486/agma202315.
39. Rodríguez LPM, Sánchez PAS. Social appropriation of knowledge applying the knowledge management methodology. Case study: San Miguel de Sema, Boyacá. AG Managment 2023;1:13-13. https://doi.org/10.62486/agma202313.
40. S. Gauthier, P. Rosa-Neto, J. A. Morais, and C. Webster, ``World Alzheimer report 2021: Journey through the diagnosis of dementia,'' Alzheimer's Disease Int., London, U.K., 2021. [Online]. Available: https://www.alzint.org/u/World-Alzheimer-Report-2021.pdf
41. Serra S, Revez J. As bibliotecas públicas na inclusão social de migrantes forçados na Área Metropolitana de Lisboa. Advanced Notes in Information Science 2023;5:49-99. https://doi.org/10.47909/978-9916-9906-9-8.50.
42. Solano AVC, Arboleda LDC, García CCC, Dominguez CDC. Benefits of artificial intelligence in companies. AG Managment 2023;1:17-17. https://doi.org/10.62486/agma202317.
43. Tanveer M, Richhariya B, Khan R, Rashid A, Khanna P, Prasad M, Lin C (2020) Machine learning techniques for the diagnosis of Alzheimer’s disease: a review. ACM Transac Multimedia Comput Commun Appl (TOMM) 16(1S):1–35.
44. Yamanakkanavar, N.; Choi, J.Y.; Lee, B. MRI Segmentation and Classification of Human Brain Using Deep Learning for Diagnosis of Alzheimer’s disease: A Survey. Sensors 2020, 20, 3243.
FINANCING
The authors did not receive funding for the development of this research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Mathews Emmanuel, J. Jabez.
Research: Mathews Emmanuel, J. Jabez.
Writing-original draft: Mathews Emmanuel, J. Jabez.
Writing-review and proof editing: Mathews Emmanuel, J. Jabez.