Category: STEM (Science, Technology, Engineering and Mathematics)
ORIGINAL
Reduction of the Wetting Collapse of Sandy Gypseous Soil by Using Microbial-Induced Calcite Precipitation
Reducción del colapso por humectación del suelo gipsoso arenoso mediante la precipitación de calcita inducida por microbios
Hadeel S. Sulaiman1 *, Muayad A. Al-Sharrad1 *, Idham A. Abed2 *
1Department of Civil Engineering, University of Anbar, Ramadi, Iraq.
2College of Agriculture, University of Anbar, Ramadi, Iraq.
Cite as: Sulaiman HS, Al-Sharrad MA, Abed IA. Reduction of the Wetting Collapse of Sandy Gypseous Soil by Using Microbial-Induced Calcite Precipitation . Salud, Ciencia y Tecnología - Serie de Conferencias. 2024; 3:817. https://doi.org/10.56294/sctconf2024817
Submitted: 19-01-2024 Revised: 01-04-2024 Accepted: 01-06-2024 Published: 02-06-2024
Editor: Dr.
William Castillo-González ![]()
Note: Paper presented at the 3rd Annual International Conference on Information & Sciences (AICIS’23).
ABSTRACT
Microbial-induced carbonate precipitation (MICP) is a promising technology for cementing sandy soils, improving ground, repairing concrete cracks, and remediating contaminated land. The aim of this research is to implement this technology in mitigating wetting collapse of Ramadi sandy gypseous soil which has a gypsum content of about 35 %. To achieve this aim, the urease-producing bacterial strain Bacillus Megaterium SI was used and treated soil specimens were prepared. The preliminary results showed a well-defined bacterium activity with a precipitated calcite of 13-16,5 % by the end of the first week. The results of the collapsibility test showed that increasing cementation solution molarity from 0,25M to 1M lowered the wetting strain and total strain caused by both loading to 100 kPa and wetting by about 75 %. Therefore, the MICP demonstrates the potential to mitigate the wetting collapse of the sandy gypseous soil despite its high gypsum content.
Keywords: MICP; Gypseous Soils; Collapsibility.
RESUMEN
La precipitación de carbonatos inducida por microbios (MICP) es una tecnología prometedora para cementar suelos arenosos, mejorar el terreno, reparar grietas de hormigón y remediar terrenos contaminados. El objetivo de esta investigación es aplicar esta tecnología para mitigar el colapso por humedecimiento del suelo gipsoso arenoso de Ramadi, que tiene un contenido de yeso de aproximadamente el 35 %. Para lograr este objetivo, se utilizó la cepa bacteriana productora de ureasa Bacillus Megaterium SI y se prepararon muestras de suelo tratado. Los resultados preliminares mostraron una actividad bacteriana bien definida con una calcita precipitada del 13-16,5 % al final de la primera semana. Los resultados de la prueba de colapsabilidad mostraron que el aumento de la molaridad de la solución de cementación de 0,25M a 1M redujo la deformación de humectación y la deformación total causada tanto por la carga a 100 kPa como por la humectación en aproximadamente un 75 %. Por lo tanto, el MICP demuestra el potencial para mitigar el colapso por humectación del suelo gipsoso arenoso a pesar de su alto contenido en yeso.
Palabras clave: MICP; Suelos Giposos; Colapsabilidad.
INTRODUCTION
Most gypseous soils demonstrate sudden volumetric changes upon wetting, due to the dissolution of gypsum which causes uneven settlement or collapsing. Consequently, engineering constructions on gypseous soil are risky where several problems such as crack induction, tilting or differential settlement, and even failure may occur. Gypseous soils are widely distributed in arid and semi-arid areas of the world such as the Arabian peninsula, Russia, Armenia, the United States, Iraq, Iran, and Spain.(1,2)
In general, collapsible soils have porous texture with high void ratio and relatively low densities. During a dry state, they possess high recorded strength, but they are susceptible to large reductions in void ratio upon wetting which leads to failure.(3) The Collapse mechanism can be explained by the initial collapse of the metastable texture of soil due to the dissolution of gypsum when the soil is subjected to the wetting condition by which bonds between grains are broken down.(4) Then, the soil particles rearrange into a denser state of packing accompanied by collapsing as the dissolved gypsum leaches out.(5)
So far, soil stabilization with cement is a comprehensively researched treatment technique and has been widely used to improve soil properties under engineering constructions, such as foundations and pavements. Nevertheless, the utilization of cement to treat soils with large gypsum contents is prone to sulfate attack.(6,7,8)
Microbial-induced carbonate precipitation (MICP) is one of the recent and promising techniques for soils stabilization.(9,10,11,12,13,14,15) In this technique, urease-producing bacteria are typically used and a bacterial suspension and a cementation solution are poured or mixed with the soil. Calcium carbonate is produced by decomposition of urea by the urease enzyme produced by the bacterium.(10,16)
Various bacterial strains have been used successfully for the calcite precipitation purpose. Bacillus megaterium and pasteurii are examples of the mostly used bacterial strains due to their superior characteristics such as their resistance to natural conditions. For instance, Bacillus megaterium is a bacterial classified as producing blackboards, which enables them to be able to coexist in harsh conditions such as high temperature, hydrocarbon pollutants, and also when the percentage of drought increases.(17)
In the MICP process, urea is hydrolyzed by microbial urease to form NH4+ and CO32–. The resulting CO32– react with Ca2+ to form CaCO3, which can be employed as a cementation material to bind the geomaterials together to the extent that the strength and stiffness of the material is increased. The MICP is currently proposed for a variety of applications including; soil improvement(18,19), concrete remediation(20), heavy metal removal(21), resistance to wind erosion(22), and construction material development.(23)
The current study investigates the effect of MICP on the collapse strain produced by wetting of a sandy gypseous soil.
Experimental Work and Procedures
The work represents a milestone on the use of MICP to reduce the magnitude of the one-dimensional wetting collapse of a sandy gypseous soil. The biological aspect of the work included selecting a suitable bacterial strain and preparing of bacterial suspension and cementation solution. The geotechnical aspects included preparing the soil for treatment, mixing and placing the soil in a test ring then curing, and performing wetting collapse tests in the oedometer apparatus.
Soil
The soil used in this study was collected at a depth of 0,5 m from a site within the main campus of the University of Anbar, as shown in figure 1. This study area is characterized with high contents of secondary origin gypsum, ranging from about 40 % at the ground surface to about 5 % few meters below the surface.(24) Signs of gypsum dissolution and movement, caused by various hydrological and environmental conditions, can be clearly observed on the site soil. Table 1 shows the main index properties of the soil which can be classified as a poorly graded sandy gypseous soil with silt.
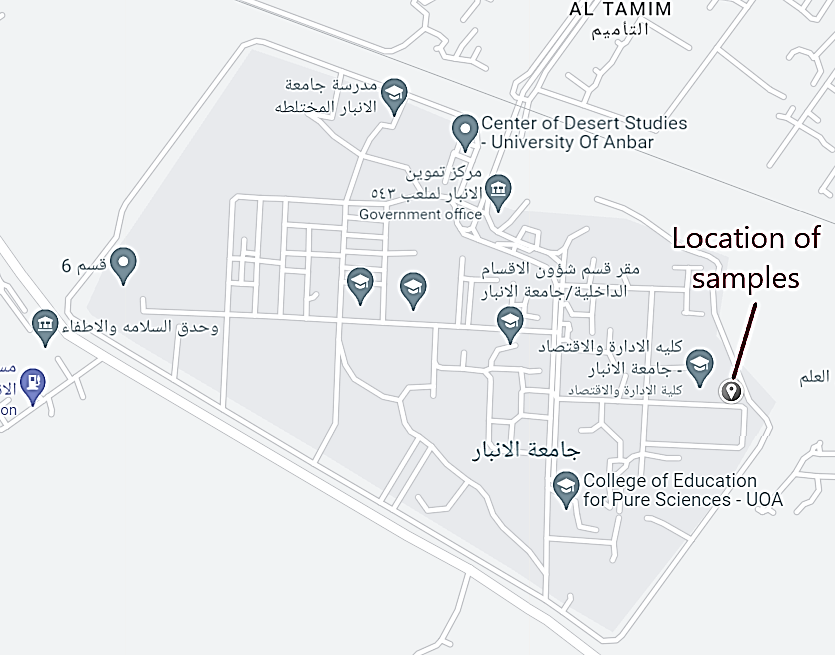
Figure 1. Location of the soil samples
|
Table 1. Index properties of the soil |
||
|
Soil property |
Value |
Standard |
|
Gravel (%) |
2,3 |
ASTM D422-2007 (25) |
|
Sand (%) |
91,7 |
|
|
Fines (%) |
6,0 |
|
|
Liquid limit (%) |
Non-plastic |
ASTM D4318- 2017 (26) |
|
Plastic limit (%) |
Non-plastic |
|
|
Specific gravity |
2,4 |
ASTM D854- 2014 (27) |
|
Gypsum content (%) |
35 |
BS 1377: Part 3 (1990) (28) |
|
Soil classification (USCS) |
Poorly graded sand with silt (SP-SM) |
ASTM D2487-17 (29) |
|
Maximum dry unit weight (kN/m3) |
15,27 |
ASTM D698- 2012 (30) |
|
Optimum moisture content (%) |
9,5 |
|
Bacteria suspension and cementation solution
Activation of Bacteria
The bacterium used in this work was Bacillus Megaterium which was initially isolated and classified by (31) from local soils at the College of Agriculture of the University of Anbar. Bacillus megaterium is a rod-like, Gram-positive, mainly aerobic, spore forming bacterium found in widely diverse habitats.(32,33) It has a cell length up to 100 µm and a diameter of 0,1 µm, which is quite large for bacteria.(34)
The strain was initially preserved in glycerol at a temperature of 4 °C. A sample of the bacterium was activated in agar medium containing 2 g yeast extract, 1g (NH4)2SO4, and 2g agar in 100 ml tris buffer. The original pH level of the solution was modified to 9 and the solution was autoclaved at 121 °C before use. After the medium cooled to about 40 °C, it was poured into Petri dishes and left to solidify, as shown in figure 2. The dishes were then inspected visually for bacteria growth. The activated isolates were cultured by striking inside a fresh petri dish. Subsequently, the dishes were placed in the incubator at 30°C for 48 hours.
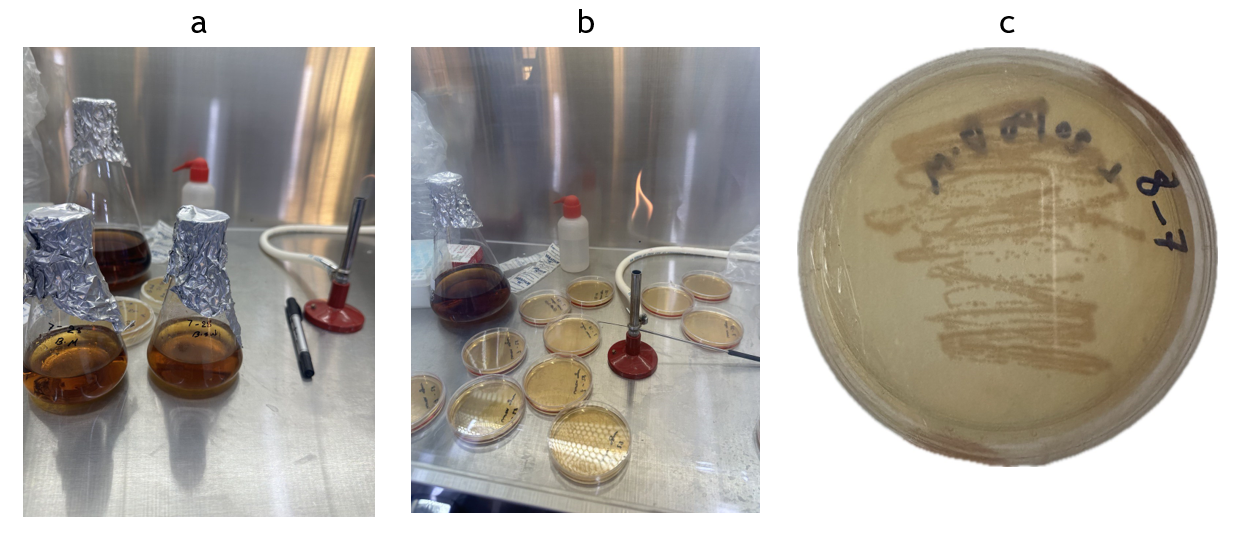
Figure 2. Preparation of the bacterial suspension: a. Preparation of liquid and solid nutrient media. b. Petri dishes containing the medium. c. Bacterial growth inside a Petri dish
Preparation of Bacterial Suspension
The liquid medium consisted of 500 ml (pH 9,0) Tris buffer, 5g (NH4)2SO4, and 10 g yeast extract was autoclaving at 121 °C before use. A 100 ml of this solution was transferred into a 250 ml flask. From the surface of the agar plate with highest concentration of single colonies, a single colony was picked up by loop and dipped into the 100 ml flask and stirred for 1 min. Afterwards, the flask was shaken in a shaking incubator at 180 rpm, 30 °C for 48 hours. Then, the bacteria were inoculated in a conical flask at a volumetric ratio of 1:100 of the bacterial solution to the culture medium and placed in a shaking incubator for 48 hours at a temperature of 30 °C.
Preparation of Cementation Solution
The cementation solution was prepared by mixing equal proportions of 0,5M of calcium chloride (CaCl2), and urea (CO(NH2)2), as recommended by (35,36). These were the main compounds of the solution and to improve the quality of the cementation solution, trace nutrients were added. In addition, the solution included: 2,12 g sodium bicarbonate NaHCO3, 10 g ammonium chloride NH4Cl, and 3 g nutrient broth, were added per liter of deionized water.
Calcite Precipitation test
In order to gain an insight into the effect of bacterial suspension to cementation solution ratio on the amount calcite precipitation, three mixing ratios were selected; 1:2, 1:1, and 2:1. Three sterilized centrifuge tubes, each of 15 ml volume were used. The liquids in these tubes were then mixed thoroughly with a vortex for few minutes. The tubes with the liquids were subsequently placed in a non-shaking incubator at 30 °C. The calcite precipitation process was monitored by inspecting the tubes frequently. It was possible to observe the calcite precipitation with the naked eye after 30 - 36 hrs, as shown in figure 3. The precipitated carbonates were first collected on Whatman No. 1 filter paper by filtration, then washed with sterile distilled water, air-dried at 60 C for 24 h, weighed, and analyzed for its chemical constituents. Similar procedure can be found in (16).
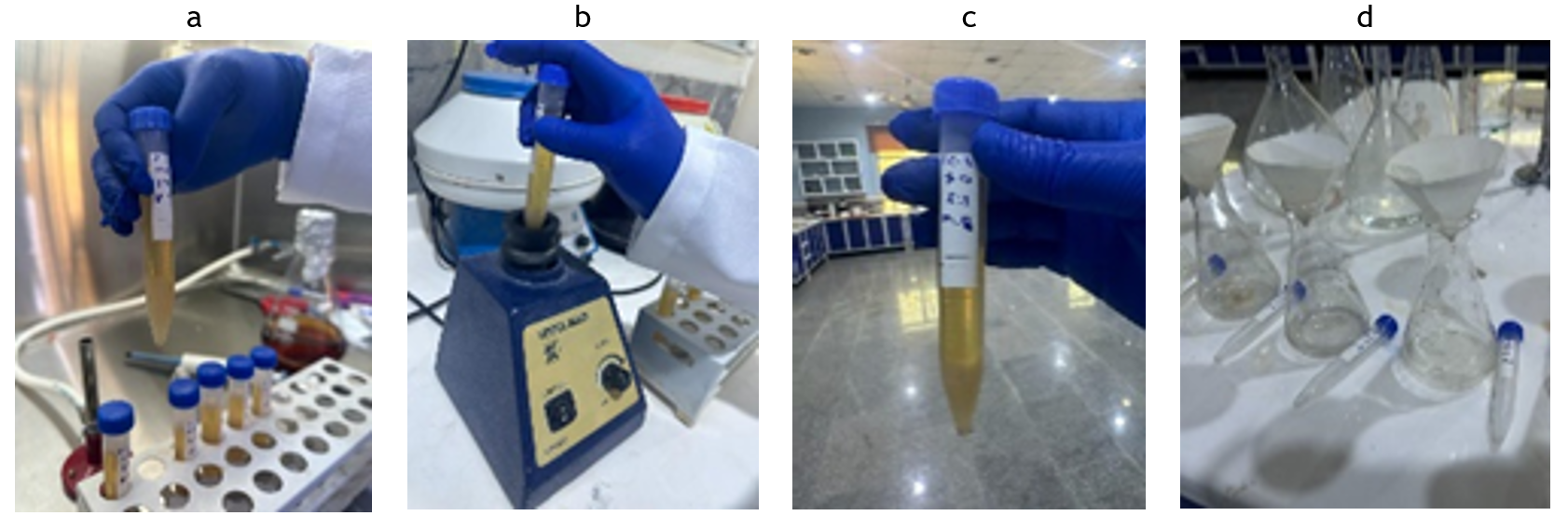
Figure 3. Calcite precipitation: a. Mixing the solutions b. Shaking with vortex. c. Calcite precipitation inside a tube d. Collection of calcium carbonates on a filter paper
Urease Enzyme Activity Assay
A 40 % urea solution was prepared by dissolving 40 grams of dry urea powder in 100 ml of distilled water. A 50 ml of the filtrated solution, taken by syringe filter, was taken and added to a 950 ml of a urea agar base medium, with the pH adjusted to 6,8. Then the resulting solution was poured into dishes to conduct microbial culture, following solidification. The substances were incubated at 30 oC until color changed from yellow to pink in response to change in the pH, depending on the amount of enzyme hydrolyzing and the accumulation of ammonia.(37,38) The result of this test was positive, as shown in figure 4, indicating that the bacterium was able to produce the enzyme. The magnitude of the urease activity, expressed in µU NH4 + N g −1 of dry soil, was estimated by (31) using the method outlined in (39). In this method, 5 g of fresh soil was added to 2,5 ml of a 0,72M urea solution and 20 ml of a 0,1M borate solution, pH 10. The combination was then incubated for two hours at 37°C. Subsequently, the released ammonia was extracted with sodium dichloroisothiorate and measured spectrophotometrically at 625 nm,(31) found an activity of 9,32 µU NH4 + N g −1 of dry soil.
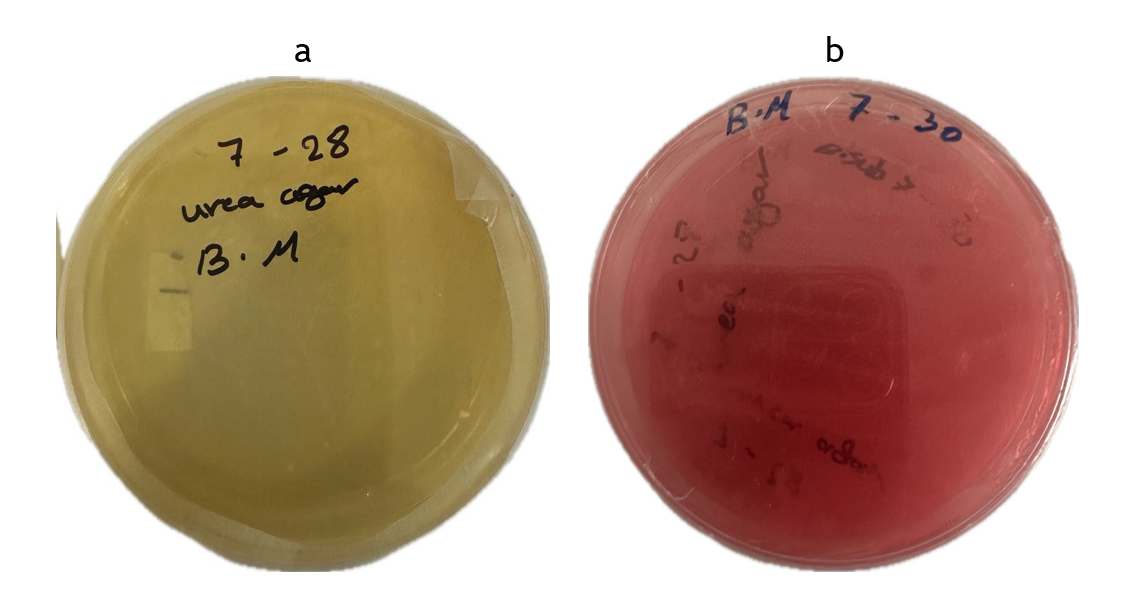
Figure 4. Color change of medium during a urease test. a. Before culturing, b. After culturing
Density (concentration) of the bacterial suspension
The bacteria concentration (OD600) is one of the important factors controlling the MICP process.(40) The bacterial concentration depends on several factors such as inoculum concentration, incubation temperature, metabolic state of the inoculum used. The optical density (concentration) of the bacterial suspension was measured with a spectrophotometer at 600 nm wavelength, OD600,(41) and was equal to 1,810, so the bacterial suspension was ready to be added to the soil. The concentration of bacteria cells suspended in the growth medium was calculated by Eq. 1, given by (42) as cited in (43) , as:
Y=8,59×107 ×Z1,3627. (1)
where Z is reading at OD600, and Y is the concentration of cells/ml.
According to Eq 1, the concentration of the bacterial suspension was 19,28 * 107 cells/ml.
Sample Preparation
The effect of induced calcite on the magnitude of the wetting collapse of the gypseous soil was investigated by performing a one-dimensional collapse test in the oedometer equipment. The test was performed in accordance with the test method B of the(44). Initially, a sample of the field soil was brought to the lab, dried and sieved on No.4 sieve (4,75mm).
To characterize the soil, the values of the maximum dry unit weight and the optimum moisture content, were obtained from the standard compaction test. To treat a soil specimen with certain magnitudes of bacterial and cementation solutions, 60 g of oven-dried soil was mixed with 15 ml bacterial solution, then molded gently inside special oedometer ring (5,32 cm in diameter and 2,04 cm in height). Two identical samples were prepared, one for the collapse test and the second for the calcite test, for each treatment period. The specimens were prepared at a dry unit weight of 13,23 kN/m3 which was purposefully less than the maximum dry unit weight (i.e., 15,27 kN/m3, table 1) and a liquid content (i.e., bacterial suspension) of 25 % which was much higher than the optimum water content (i.e., 9,5 %, table 1). The lower unit weight values and higher bacterial suspension content promote better calcite production and precipitation.
To avoid soil disturbance while immersing the cementation solution, a layer of fine filter was mounted on the top of the specimen. Also, to prevent fine soil particles smear from the specimen, another layer of the filter was placed below the specimen. Furthermore, a perforated plastic covers with evenly distributed holes were placed above and below the specimen for uniform distribution of the cementation solution within the specimen. Three containers of cementation solution were prepared at three concentrations of 0,25, 0,5 and 1M, in equal proportions of urea and calcium chloride, and the previously prepared specimens were immersed for 7 and 14 days (figure 5). The containers are equipped with an air pump to provide suitable conditions for the bacteria.

Figure 5. Stabilized soil samples: a. samples after preparation. b. submerged samples in a cementation solution
Loading and Wetting Process
Following the treatment stage, the oedometer ring which contained the specimen was mounted in the oedometer mold, as shown in figure 6. The vertical pressure on the specimen was applied at a load incremental ratio of 1 up to the intended soaking pressure and the change of specimen’s height was recorded correspondingly. Soaking was initiated once the change in specimen’s height ceased after the last loading increment. All the specimens were soaked under a constant vertical pressure of 100 kPa. From practical point of view, this level of loading is considered moderate, given the collapsing nature of the soil.

Figure 6. Collapse test setup
The results of the collapsibility tests were interpreted in terms of effective normal stress and axial strain, where the latter was calculated in this work as:
ɛ=(h0-h)/h0*100. (2)
where h0 is the initial height of the specimen. The wetting strain was calculated as
ɛc=(h1-h2)/h0*100. (3)
where h1 and h2 are the specimen’s heights before and after wetting, respectively, at a given stress level.
Calcite content test
In this research, the calcite content of treated soil was determined by the acid wash method described in [45]. This test was conducted on identical treated specimens before and after soaking. In each test, a sample of 5 g was taken after mixing the material thoroughly. A 20 ml of 1M HCl was added, and left for 1 hour to dissolve all the calcite. Then the suspension was filtered on a dry medium filter paper of known mass. The remaining material was then washed with distilled water, and then dried for 24 hours at a temperature of 105 oC. The calcite content, CC%, is calculated as:
CC%=100-B\A*100. (4)
where A and B are the masses of the oven-dried filter paper before and after the filtration.
RESULTS
Figures 7 and 8 present stress-strain response during collapse tests performed on specimens treated with bacterial and cementation solutions, at the ages of 7 and 14 days, respectively. For the loading stage preceding soaking, the untreated specimen exhibited lesser compressional strain compared to those treated and cured for 7 days (figure 7), whereas it exhibited greater strain compared to those treated and cured for 14 days (figure 9). This presumably attributed to chemical interaction of the added solutions and the gypsum of the soil. Undesirable partial gypsum dissolution was expected due to the availability of substances in the solutions, which can dissolve gypsum. The partial dissolution of gypsum would leave the soil with larger voids and result in greater axial strains. Conversely, the production of calcium carbonate, which is time dependent, gradually fills the voids and bridges the soil particles. The production of the calcium carbonate was confirmed experimentally, where the mass percentage of calcite content was 13-16,5 %.
Interestingly, the produced calcium carbonate contributed effectively in mitigating wetting strains even at ages of as early as 7 days, as shown in figures 7 and 8. The magnitudes of wetting strains and the total strains caused by loading to 100 kPa then wetting, are further analyzed in figures 9 and 10, respectively. These strains clearly decreased with increasing cementation molarity and age of curing. For instance, with increasing molarity to 1M, the wetting strain deceased from about 3 % to just about 0,65 % and 0,7 % at the ages 7 and 14 days, respectively. Similarly, the total strain deceased from about 5,6 % to 4 % and 1,3 % at these ages.
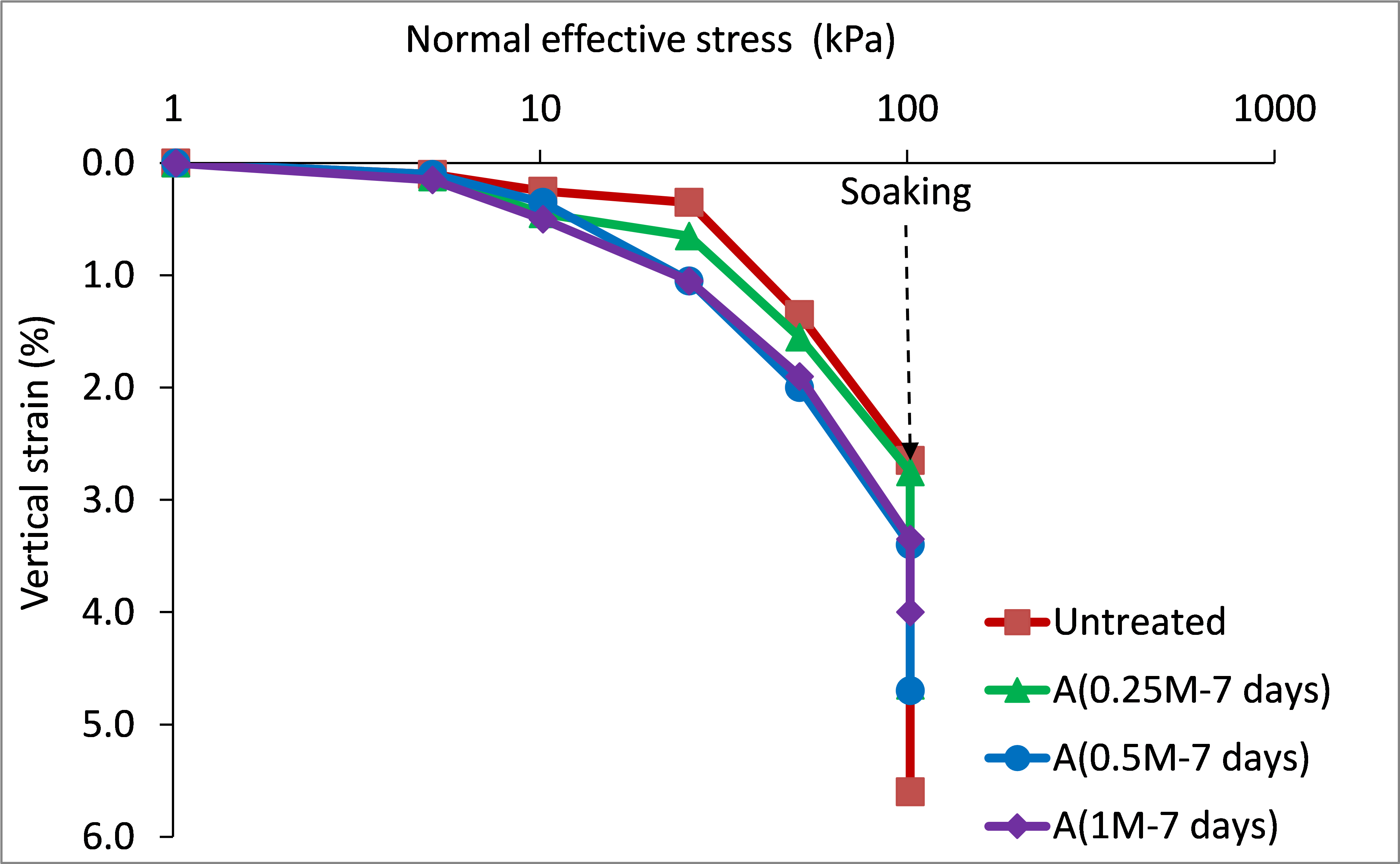
Figure 7. Oedometric stress-strain behavior of 7 days cured specimens
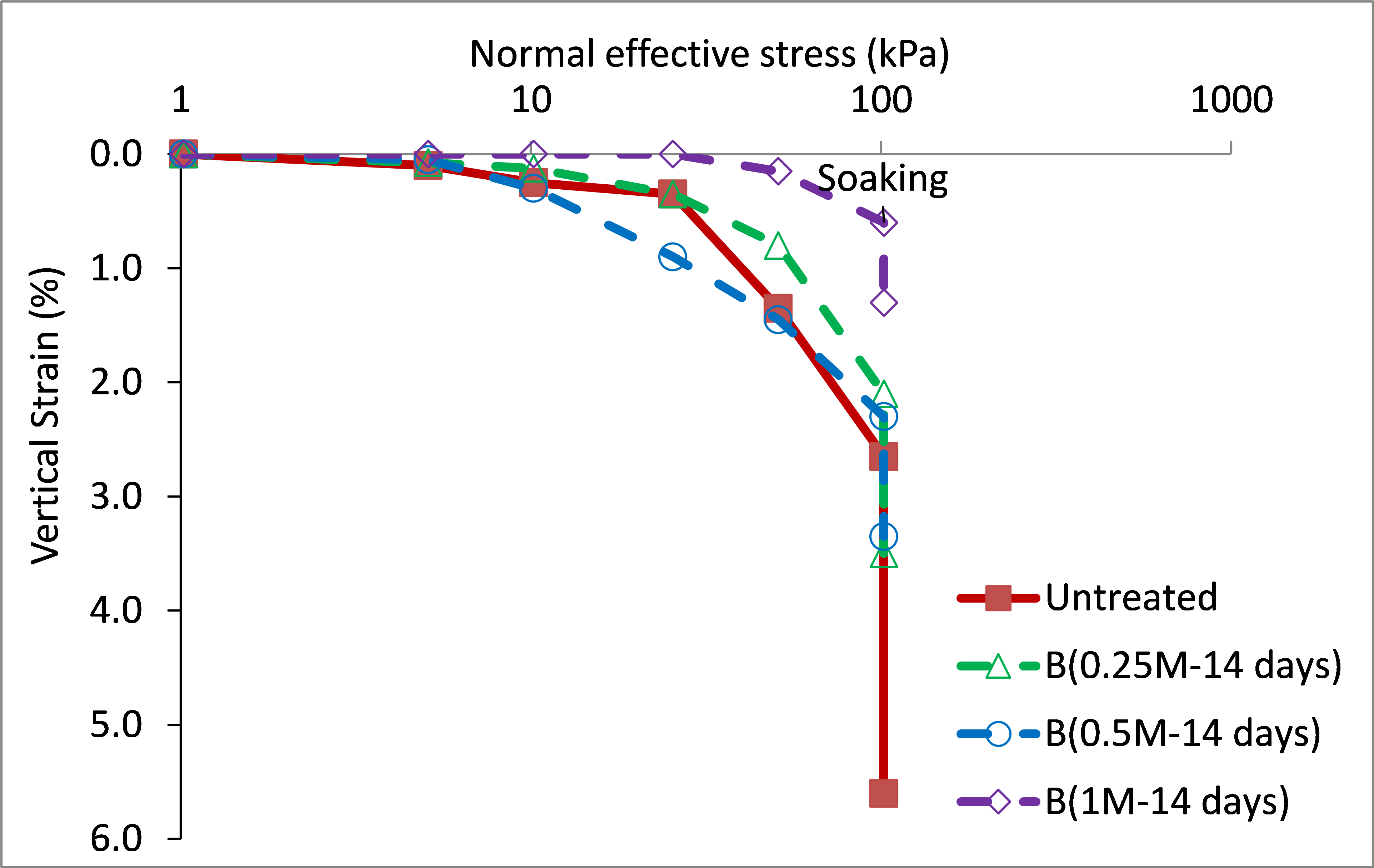
Figure 8. Oedometric stress-strain behavior of 14 days cured specimens
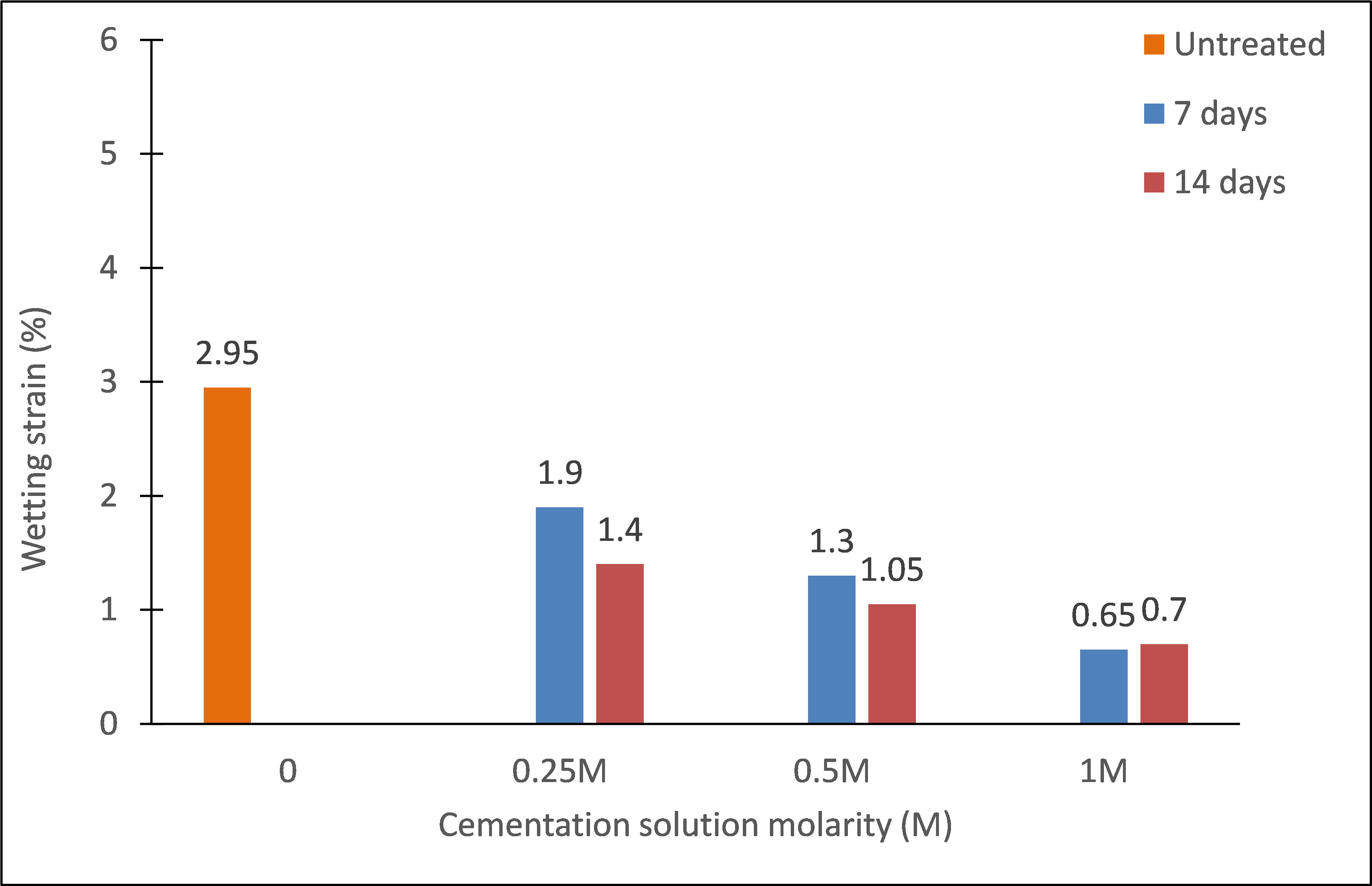
Figure 9. Effect of cementation solution molarity on wetting collaspe strain
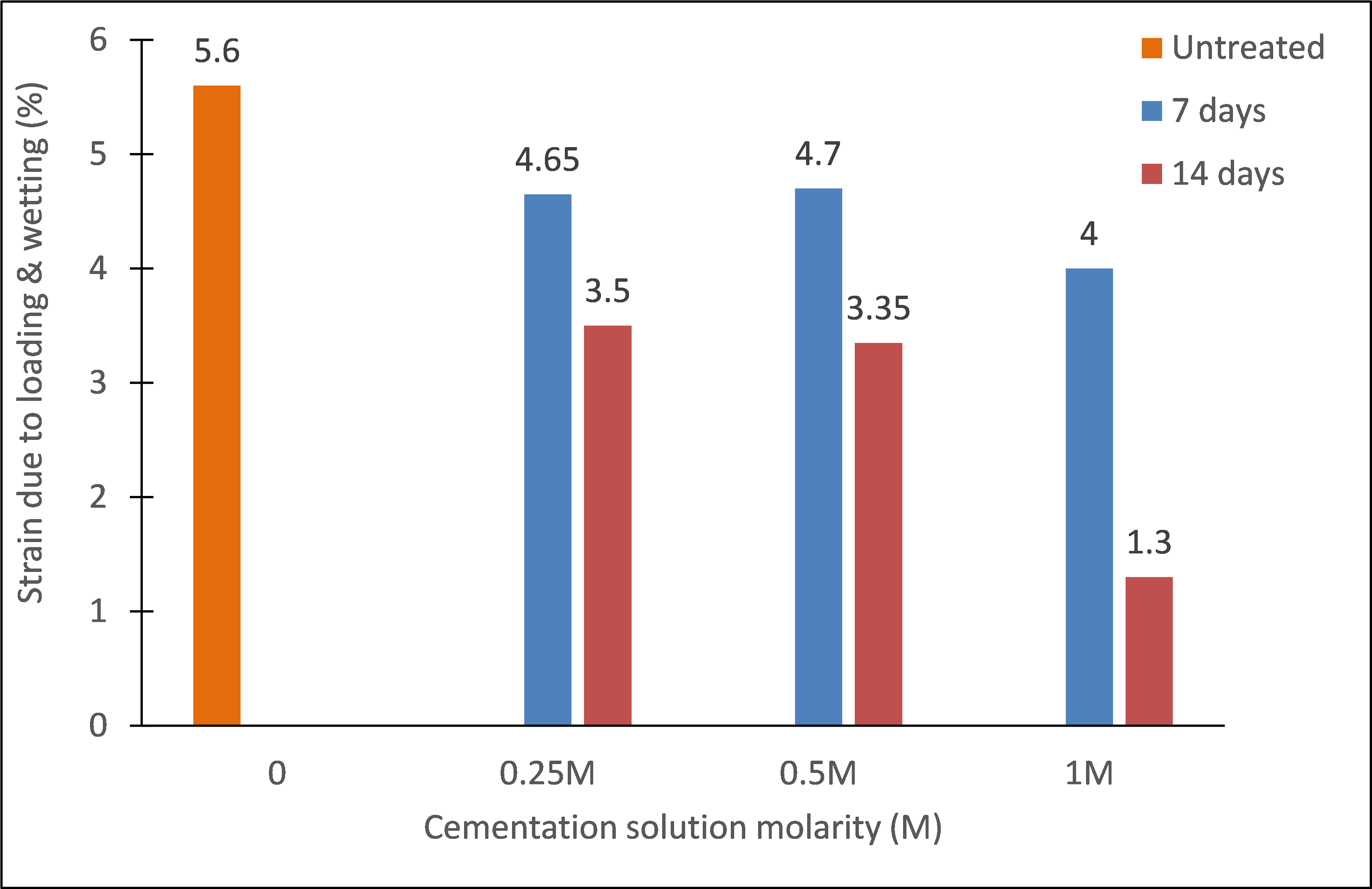
Figure 10. Effect of cementation solution molarity on loading & wetting strain
CONCLUSION
The current work presents a number of results representing early attempts on the use of microbial induced carbonate precipitation to mitigate collapsibility of a sandy gypseous soil. In particular, the soil was mixed with Megaterium suspension, molded, then immersed in a cementation solution having calcium chloride (CaCl2), and urea (CO(NH2)2), of different molarities. The urease activity test confirmed that the bacterium has a noticeable activity. This was further confirmed by the calcite content test which resulted in a considerable amount of calcite of 13 to 16 %. The collapsibility test indicated that the MICP can be effectively used to reduce collapse caused by water immersion, even for soils with high gypsum contents. The results showed that increasing cementation solution molarity from 0.25M to 1M, led to a reduction in the wetting strain and total strain by almost 75 %.
REFERENCES
1. T. G. Boyadgiev and W. H. Verheye, “Contribution to a utilitarian classification of gypsiferous soil,” Geoderma, vol. 74, no. 3–4, pp. 321–338, 1996.
2. S. Casby-Horton, J. Herrero, and N. A. Rolong, “Gypsum soils—Their morphology, classification, function, and landscapes,” Adv. Agron., vol. 130, pp. 231–290, 2015.
3. A. Jotisankasa, “Collapse behaviour of a compacted silty clay.” University of London London, UK, 2005.
4. M. Y. Fattah, I. H. Obead, and H. A. Omran, “A study on leaching of collapsible gypseous soils,” Int. J. Geotech. Eng., vol. 16, no. 1, pp. 44–54, “A study on leaching of collapsible gypseous soils,” Int. J. Geotech. Eng., vol. 16, no. 1, pp. 44–54, 2022, doi: 10.1080/19386362.2019.1647664.
5. G. Bolzon, “Collapse mechanisms at the foundation interface of geometrically similar concrete gravity dams,” Eng. Struct., vol. 32, no. 5, pp. 1304–1311, 2010.
6. S. S. Razouki, R. R. Al-Omari, I. H. Nashat, H. F. Razouki, and S. Khalid, “The problem of gypsiferous soils in Iraq,” in Proceeding of the symposium on gypsiferous soils and their effect on structures, NCCL, 1994, pp. 7–33.
7. S. S. Razouki and O. A. El-Janabi, “Decrease in the CBR of a gypsiferous soil due to long-term soaking,” Q. J. Eng. Geol. Hydrogeol., vol. 32, no. 1, pp. 87–89, 1999.
8. S. A. Khattab, “Effect of gypsum on strength of cement treated granular soil and untreated soil.” University of Mosul Iraq, 1986.
9. J. T. DeJong, M. B. Fritzges, and K. Nüsslein, “Microbially induced cementation to control sand response to undrained shear,” J. Geotech. geoenvironmental Eng., vol. 132, no. 11, pp. 1381–1392, 2006.
10. A. Al Qabany, K. Soga, and C. Santamarina, “Factors affecting efficiency of microbially induced calcite precipitation,” J. Geotech. Geoenvironmental Eng., vol. 138, no. 8, pp. 992–1001, 2012.
11. A. Al Qabany and K. Soga, “Effect of chemical treatment used in MICP on engineering properties of cemented soils,” in Bio-and Chemo-Mechanical Processes in Geotechnical Engineering: Géotechnique Symposium in Print 2013, ICE Publishing, 2014, pp. 107–115.
12. L. Cheng, R. Cord-Ruwisch, and M. A. Shahin, “Cementation of sand soil by microbially induced calcite precipitation at various degrees of saturation,” Can. Geotech. J., vol. 50, no. 1, pp. 81–90, 2013.
13. Z. S. Hadi and K. A. Saeed, “Effect of microbial-induced calcite precipitation (MICP) on the strength of soil contaminated with lead nitrate,” J. Mech. Behav. Mater., vol. 31, no. 1, pp. 143–149, 2022, doi: 10.1515/jmbm-2022-0016.
14. A. D. Salman, “Effect of microbial induced calcite precipitation and nanomaterials techniques on improving the behavior of gypseous soils,” Univ. Baghdad, Dep. Civ. Eng., 2021.
15. A. D. Almurshedi and M. Karkush, “Experimental and numerical modeling of load settlement behavior of gypseous soils improved by MICP,” in Smart Geotechnics for Smart Societies, CRC Press, 2023, pp. 583–589.
16. N. K. Dhami, M. S. Reddy, and A. Mukherjee, “Biomineralization of calcium carbonate polymorphs by the bacterial strains isolated from calcareous sites,” J. Microbiol. Biotechnol., vol. 23, no. 5, pp. 707–714, 2013.
17. L. Wang, T. L. K. Yeung, A. Y. T. Lau, D. C. W. Tsang, and C.-S. Poon, “Recycling contaminated sediment into eco-friendly paving blocks by a combination of binary cement and carbon dioxide curing,” J. Clean. Prod., vol. 164, pp. 1279–1288, 2017.
18. S. Liu, K. Du, K. Wen, W. Huang, F. Amini, and L. Li, “Sandy soil improvement through microbially induced calcite precipitation (MICP) by immersion,” J. Vis. Exp., vol. 2019, no. 151, 2019, doi: 10.3791/60059.
19. B. C. Martinez et al., “Experimental optimization of microbial-induced carbonate precipitation for soil improvement,” J. Geotech. Geoenvironmental Eng., vol. 139, no. 4, pp. 587–598, 2013.
20. X. Sun, L. Miao, L. Wu, and H. Wang, “Theoretical quantification for cracks repair based on microbially induced carbonate precipitation (MICP) method,” Cem. Concr. Compos., vol. 118, p. 103950, 2021.
21. H. Bai et al., “Microbially-induced calcium carbonate precipitation by a halophilic ureolytic bacterium and its potential for remediation of heavy metal-contaminated saline environments,” Int. Biodeterior. Biodegradation, vol. 165, p. 105311, 2021.
22. Z. Wang, N. Zhang, J. Ding, C. Lu, and Y. Jin, “Experimental study on wind erosion resistance and strength of sands treated with microbial-induced calcium carbonate precipitation,” Adv. Mater. Sci. Eng., vol. 2018, 2018.
23. S. E. Lambert and D. G. Randall, “Manufacturing bio-bricks using microbial induced calcium carbonate precipitation and human urine,” Water Res., vol. 160, pp. 158–166, 2019.
24. M. A. Al-Sharrad, “Collapsibility and leaching behavior of an artificial sandy gypseous soil,” Bull. Eng. Geol. Environ., vol. 82, no. 12, p. 445, 2023.
25. ASTM D422. (2007). Standard test method for particle-size analysis of soils (ASTM International). www.astm.orgNo Title.
26. “ASTM D4318. (2017). Standard test methods for liquid limit, plastic limit, and plasticity index of soils: Vol. 04.08. ASTM International. http://www.astm.org.No Title”.
27. “ASTM D854. (2014). Standard Test Methods for specific gravity of sil solids by water pycnometer. ASTM International.No Title”.
28. M. Gingras, “One hundred years of helicene chemistry. Part 3: applications and properties of carbohelicenes,” Chem. Soc. Rev., vol. 42, no. 3, pp. 1051–1095, 2013.
29.“ASTM D2487-17 Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System) ASTM International, West Conshohocken, PA (2017), 10.1520/D2487-17”.
30. ASTM D698. (2012). Standard test method for laboratory compaction characteristics of soil using standard effort (12 400 ft-lbf/ft3 (600 kN-m/m3)). (ASTM International). www.astm.orgNo Title.
31. K. kudury S. A. I. M. B, “MICROBIAL FERTILIZERS EXISTENCE AND ITS RELATIONSHIP TO HEAVY METALS IN SOME SUSTAINABLE AGRICULTURAL FIELDS IN ANBAR GOVERNORATE,” Anbar J. Agric. Sci., vol. 21, no. 1, 2023.
32. P. De Vos and G. M. Garrity, Bergey’s manual of systematic bacteriology. Springer, 2009.
33. P. S. Vary et al., ‘Bacillus megaterium—from simple soil bacterium to industrial protein production host,’ Appl. Microbiol. Biotechnol., vol. 76, pp. 957–967, 2007.
34. S. R. R. Tadi, G. Nehru, and S. Sivaprakasam, “Metabolic Engineering of Bacillus megaterium for the Production of β-alanine,” Biotechnol. Bioprocess Eng., vol. 27, no. 6, pp. 909–920, 2022.
35. Wei-Soon Ng, Min-Lee Lee, and Siew-Ling Hii, “An Overview of the Factors Affecting Microbial-Induced Calcite Precipitation and its Potential Application in Soil Improvement,” Int. J. Civ. Environ. Eng., vol. 6, no. 2, pp. 188–194, 2012, [Online]. Available: https://pdfs.semanticscholar.org/dc1f/0edb47ecdc6b4a1ce6b46ab6a4114ae60503.pdf
36. M. Nemati, E. A. Greene, and G. Voordouw, “Permeability profile modification using bacterially formed calcium carbonate: comparison with enzymic option,” Process Biochem., vol. 40, no. 2, pp. 925–933, 2005.
37. V. Achal, A. Mukherjee, P. C. Basu, and M. S. Reddy, “Strain improvement of Sporosarcina pasteurii for enhanced urease and calcite production,” J. Ind. Microbiol. Biotechnol., vol. 36, no. 7, pp. 981–988, 2009.
38. J. G. Collee, Mackie & McCartney practical medical microbiology., 14th ed. / edited... Edinburgh ; Churchill Livingstone, 1996.
39. K. Y. Kim, D. Jordan, and G. A. McDonald, “Effect of phosphate-solubilizing bacteria and vesicular-arbuscular mycorrhizae on tomato growth and soil microbial activity,” Biol. Fertil. soils, vol. 26, pp. 79–87, 1997.
40. Q. Zhao, L. Li, C. Li, H. Zhang, and F. Amini, “A full contact flexible mold for preparing samples based on microbial-induced calcite precipitation technology,” Geotech. Test. J., vol. 37, no. 5, pp. 917–921, 2014.
41. B. Liu et al., “Potential drought mitigation through microbial induced calcite precipitation‐MICP,” Water Resour. Res., vol. 57, no. 9, p. e2020WR029434, 2021.
42. S. K. Ramachandran, V. Ramakrishnan, and S. S. Bang, “Remediation of concrete using microorganisms,” Mater. J., vol. 98, no. 1, pp. 3–9, 2001.
43. W. Wan et al., “Isolation and characterization of phosphorus solubilizing bacteria with multiple phosphorus sources utilizing capability and their potential for lead immobilization in soil,” Front. Microbiol., vol. 11, p. 752, 2020.
44. “ASTM D4546. (2014). Standard test method for one-dimensional swell or collapse of soils. (ASTM International). www.astm.orgNo Title”.
45. S.-G. Choi, S.-S. Park, S. Wu, and J. Chu, “Methods for calcium carbonate content measurement of biocemented soils,” J. Mater. Civ. Eng., vol. 29, no. 11, p. 6017015, 2017.
FINANCING
None.
CONFLICT OF INTEREST
None.
AUTHORSHIP CONTRIBUTION
Conceptualization: Hadeel S. Sulaiman, Muayad A. Al-Sharrad, Idham A. Abed.
Research: Hadeel S. Sulaiman, Muayad A. Al-Sharrad, Idham A. Abed.
Writing - original draft: Hadeel S. Sulaiman, Muayad A. Al-Sharrad, Idham A. Abed.
Writing - revision and editing: Hadeel S. Sulaiman, Muayad A. Al-Sharrad, Idham A. Abed.