Category: Health Sciences and Medicine
REVIEW
Use of the Zebrafish Model as a tool to evaluate the anti- inflammatory and antioxidant activity of molecules. Literature Review
Uso del Modelo del Pez Cebra como herramienta para evaluar la actividad antiinflamatoria y antioxidante de moléculas. Revisión de literatura
Cristina Arteaga1 ![]() *, Luis Felipe Contreras2 *, Ruth Borja3 *, Carolina Campoverde4 *, Alberto Bustillos4
*, Luis Felipe Contreras2 *, Ruth Borja3 *, Carolina Campoverde4 *, Alberto Bustillos4 ![]() *
*
1Universidad Técnica de Ambato, Facultad de Ciencias de la Salud, Carrera de Nutrición y Dietética, Ambato, Ecuador.
2Universidad Técnica de Ambato, Facultad de Ingeniería Civil y Mecánica, Ambato, Ecuador.
3Universidad Técnica de Ambato, Facultad de Ciencia e Ingeniería en Alimentos y Biotecnología, Ambato, Ecuador.
4Universidad Técnica de Ambato. Facultad de Ciencias de la Salud, Carrera de Medicina, Ambato, Ecuador.
Citar como: Arteaga C, Contreras LF, Borja R, Campoverde C, Bustillos A. Use of the Zebrafish Model as a Tool to Evaluate the Anti- Inflammatory and Antioxidant Activity of Molecules. Literature Review. Salud, Ciencia y Tecnología - Serie de Conferencias. 2024; 3:793. https://doi.org/10.56294/sctconf2024793
Enviado: 11-01-2024 Revisado: 27-03-2024 Aceptado: 31-05-2024 Publicado: 01-06-2024
Editor: Dr.
William Castillo-González ![]()
ABSTRACT
Introduction: the evaluation of antioxidant and anti-inflammatory properties in biological models is crucial for advancing pharmacological research. The zebrafish model (Danio rerio) is increasingly used due to its genetic similarity to humans and its translational relevance in drug discovery. This work synthesizes the existing literature on the use of zebrafish as a model for testing the efficacy of various substances with antioxidant and anti-inflammatory properties.
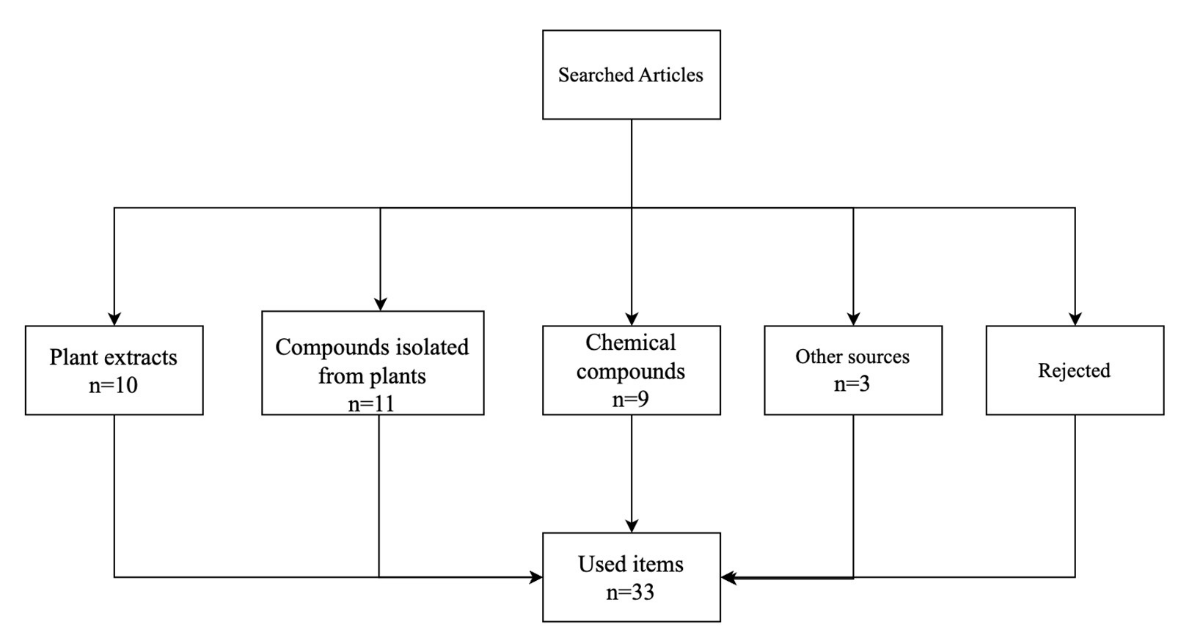
Methods: a comprehensive literature review was conducted using the Web of Science database. Search terms included “zebra fish,” “antioxidant,” “anti-inflammatory,” “model,” and “Danio rerio.” Out of fifty articles initially reviewed, thirty-three met the inclusion criteria and were analyzed further. These articles were categorized based on the source of the substances tested, including plant extracts, compounds extracted from plants, chemical compounds, and other sources.
Results: the reviewed studies utilized a variety of methods to assess the antioxidant and anti-inflammatory effects in zebrafish, including observational assays and molecular techniques. The substances tested were derived mainly from plant extracts and chemical compounds. The analysis highlights significant variability in methodology and outcomes, reflecting both the versatility and the challenges of using zebrafish in this type of research.
Conclusions: the zebrafish model is a valuable tool for studying antioxidant and anti-inflammatory properties, offering several advantages including genetic tractability, cost-effectiveness, and the ability to observe phenotypic changes in vivo. However, standardization of methodologies and a deeper understanding of the model’s limitations are essential for maximizing its utility in biomedical research. This review provides a foundation for future studies aiming to optimize zebrafish use in evaluating therapeutic agents.
Keywords: Zebrafish; Anti-Inflammatory; Antioxidant; Model.
RESUMEN
Introducción: la evaluación de propiedades antioxidantes y antiinflamatorias en modelos biológicos es crucial para avanzar en la investigación farmacológica. El modelo de pez cebra (Danio rerio) se utiliza cada vez más debido a su similitud genética con los humanos y su relevancia translacional en el descubrimiento de fármacos. Este trabajo sintetiza la literatura existente sobre el uso del pez cebra como modelo para probar la eficacia de diversas sustancias con propiedades antioxidantes y antiinflamatorias.
Métodos: se realizó una revisión exhaustiva de la literatura utilizando la base de datos Web of Science. Los términos de búsqueda incluyeron “pez cebra,” “antioxidante,” “antiinflamatorio,” “modelo,” y “Danio rerio.” De cincuenta artículos inicialmente revisados, treinta y tres cumplieron con los criterios de inclusión y fueron analizados más a fondo. Estos artículos se categorizaron según la fuente de las sustancias evaluadas, incluyendo extractos de plantas, compuestos extraídos de plantas, compuestos químicos y otras fuentes.
Resultados: los estudios revisados utilizaron una variedad de métodos para evaluar los efectos antioxidantes y antiinflamatorios en el pez cebra, incluyendo ensayos observacionales y técnicas moleculares. Las sustancias evaluadas provenían principalmente de extractos de plantas y compuestos químicos. El análisis resalta una variabilidad significativa en la metodología y los resultados, reflejando tanto la versatilidad como los desafíos de usar el pez cebra en este tipo de investigaciones.
Conclusiones: el modelo de pez cebra es una herramienta valiosa para estudiar propiedades antioxidantes y antiinflamatorias, ofreciendo varias ventajas incluyendo la tractabilidad genética, el costo-efectividad, y la capacidad de observar cambios fenotípicos in vivo. Sin embargo, la estandarización de metodologías y una comprensión más profunda de las limitaciones del modelo son esenciales para maximizar su utilidad en la investigación biomédica. Esta revisión proporciona una base para futuros estudios que buscan optimizar el uso del pez cebra en la evaluación de agentes terapéuticos.
Palabras clave: Pez Cebra; Antiinflamatorio; Antioxidante; Modelo.
INTRODUCTION
Science has always known how to take advantage of natural products to get new drugs or to use plant metabolites to obtain new compounds. Metabolites are found from several sources, one of the most used is plant sources, in addition, can be obtained from animals, and microorganisms.(1) The metabolites used in medicine are produced in the secondary metabolism of organisms, having various applications in various fields of health and food, currently powered by genetic engineering with which greater production is obtained and costs are reduced.(2) The need for new drugs to treat existing or new diseases means that new metabolites and plant-active ingredients helpful to humans are still being studied, the constant exploration of new products may present certain drawbacks such as the toxicity of the compounds, which may cause health damage, therefore a preliminary study of these compounds is necessary, together with the toxicological effects they could have on man.(3)
The evaluation of the metabolites includes the determination of the maximum tolerable dose, which allows knowing the dose at which its use is safe, in addition, the effects that could produce by its continuous use should be studied, for which several animal models are used in vivo,(4)this allows increasing the safety of products to be used by humans, in addition to an approach to the real effect of the metabolite in cells),(5) which is complemented by in vitro analysis, Cytotoxicity and its effect on the development of embryos or specific tissues are also evaluated.(6)
In vivo models acknowledge for optimization research times, avoid using humans for testing and ensure the safety of metabolites in development(7), but several organizations have a conflict with the use of animals for this purpose. However, it is important to observe the effect of new compounds in living organisms and therefore remains the most used option to test metabolites and active principles.(8)
One of the main animal models used are rodents, such as mice, mice, and rabbits, however, in recent years the zebrafish has positioned itself as a reference as an animal model,(9) because its rapid reproduction, the transparency of its skin and the subsequent direct observation of effects on their internal organs (10) are easy to manage and are 70 % homologous with the human genome and share 84 % of gene similarity associated with diseases in humans.(11) These characteristics make the zebrafish one of the best models to test metabolites, extracts, or food matrices as the quantities of compounds required are minimal and costs are reduced. It also allows observing a certain effect during all its life stages.(12) For all the above mentioned we can say that the objective of this review is to analyze the different treatments, metabolites, and results of antioxidant and anti-inflammatory activity used in zebrafish-based models for comparison and consideration of future applications in research projects.
METHOD
The present study was based on a review of scientific articles, which was carried out using Web Of Science databases. Publications were identified using search words and terms containing "zebrafish", "anti-inflammatory" and "antioxidant". In particular, the main keywords sought included "zebrafish model", "in vivo model", "oxidative stress", "inflammation" and "toxicology". A total of 50 articles were reviewed, of which 33 were used for the literature review based on the antioxidant and anti-inflammatory effect of compounds used in zebrafish models, original articles and books were included. It was verified that the revised publications do not exceed 10 years of publication. The publications were selected according to their relevance and topicality.
RESULTS
Figure 1. Summary of the number of articles reviewed
DISCUSSION
Bibliographical material
From a search in the databases Elvisier-Scopus, PubMed and Springer took 50 articles according to the subject that could fit with the bibliographic review, of these 17 articles were discarded, leaving 33 articles selected for review, The discard was given by an analysis of the procedures and tests that were carried out in each article and thus to associate the methods of analysis to determine oxidative stress and inflammation using zebrafish model.
Plant extracts
Plants are a source of nature’s most important secondary metabolites, the generation of extracts and their application in treatments or to identify new specific substances used to make medicines, the potential of these extracts is of great importance for the food and pharmaceutical industry.(14-30)
In the first phase of the metabolite revision, it was observed that in several studies plant extracts were used for both antioxidant and anti-inflammatory activity tests, five of the reviewed studies used extracts, mainly freeze-dried, Boeri et al.(14) and Nguyen T.(31) performed an extraction in organic solvents that are toxic to zebrafish, so it is necessary to remove these solvents before using the compound, while Pradeep et al.(32) used the fraction in ethanol, Conducting tests to determine oxidative stress directly with the extract; this indicates that the status of the metabolite’s final product is a factor that can influence the antioxidant activity and in vivo analysis of the model.
As non-purified compounds are used in these phases, structure, and composition analysis are required, the most used methods for this are HTPLC (high-performance thin layer chromatography), characterized by electrophoresis, inhibition of proteases and digestibility in vitro, in addition to antioxidant activity using DPPH (2,2-diphenyl-1-picrilhydrazil); Pradeep et al.(32) implemented biocompatibility and Boeri et al.(14) gastric and intestinal digestion as relevant tests.
While in tests with the zebrafish model, mortality tests were performed by electron microscope, with the measurement of the heart rate and hatching rate of fish, in addition to observing morphological changes by the same method; (Nguyen et al., 2020) and (Boeri et al., 2020) DCF-DA (dichlorodihydrofluorescein diacetate), also known as DCFH-DA, was used to measure the generation of intracellular radicals, this is a fluorescence technique based on cell staining and reaction intensity with ROS (reactive oxygen species), (Pradeep et al., 2019) real-time PCR (polymerase chain reaction) (qPCR) to identify expression of genes related to antioxidant activity, because the extract can serve as an antioxidant or as a stimulant of antioxidant activity within the individual, studied genes L-1b, IL-6, Cat, Cu-Zn, SoD;(15) also use qPCR but for specific primers 18s and hcar1. Only Poormina S, et al.(17) performed histopathological analysis of the intestine.
Arteaga and others(33) analyzed the effect on neutrophil migration as part of the antiinflammatory process by using degreased tomato sofrito extract, using neutrophil staining techniques with Leucognost smallpox kit.(15) Instead, it tracked neutrophils with fluorescence and software, just as Wang and Liu(21), used other fluorescence markers for this process.
Fertilized eggs were used in the early stages of development, with Mohamad Shariff et al.(19), Pradeep et al.(18) and Boeri et al.(14), at 1-hour post-fertilization (h.d.f),(14,16,21) between 2 and 6 h.p.f.(20) It occupied fish of 10 h.p.f, while, in the other studies embryos of 1- and 3-days postfertilization was used (d.p.f). While in oxidative stress trials only three studies used oxidants, AAPH (2,2'-azobis dihydrochloride (2-amidinopropane) with Boeri et al.(14), copper sulfate with Nguyen et al.(31) and oxazolone with Poornima et al.(17)
Isolated compounds from plants
If studies of plant compounds are available, this allows further testing with a specific compound, removing interference and accurately determining a certain effect, twelve articles were reviewed where they used one or several isolated plant compounds, without treatment or conversion, only purified.
Being already known compounds reduces the need to analyze their structure, so only five ofthem required a deep study of this type, Kim et al.(34) used HCPCP (high-performance centrifugation partition chromatography) and HPLC to analyze the loliolide you used, Xia et al.(35) used ethanol chromatography for the metabolite, while Rajasekar et al.(28) was the one that required the most tests as solubility in different polar and non-polar solvents, carbohydrate content, proteins, uranic acid and structural characterization by UV-vis spectrophotometry (ultraviolet-visible radiation), Roberto et al.(36) used eight chemical analyses, among which highlight antioxidant activity by DPPH and beta-carotene assay with chloroform, finally, Jayawardena et al.(37) used several characterizations such as polyphenols analysis, sulfate content, among others referring to his research.
Cho et al.(38) highlighted with a Western blot assay for antibodies linked to immunological activity, these were anti-bax, anti-bcl-xL, anti-PARP, anti-cleaved caspase-9 anti-phospho- NF-κB p105, anti-phospho-NF-κB p65, anti-rabbit IgG.
In the in vivo trials, fish from 7 to 9 h.p.f were used in the works of Cho et al.(38); Jayawardena et al.(37); Kim et al.(39), while Arteaga et al.(22), embryo use between 0 and 4 h.p.f as well as other authors, Issac et al.(26); Kang, Kim, et al.(25); Xia et al.(30); on the other hand, larvae of 6 d.p.f. were used in the work of Endo et al.(40) because it required fish that could withstand strong metabolites against animal metabolism, Roberto et al.(27) used fish of 7 d.p.f while Rajasekar et al.(28), was the one who used fish of greater age 15 d.p.f., mainly because the metabolite was supplied orally by means of a mixture in the food. In the fish assays, apoptosis was included in six of the eleven papers, all of which used acridine orange staining assays for cell observation; Mortality was observed using an optical microscope, measuring the heart rate of the fish. Gene expression detection was obtained by western blot, especially Cat, Sos, Nrf2, Keap1 in Jayawardena et al.(37) and ax, Bcl-xL, cleaved caspase-3, PAR in (Kim et al., 2020), these being antioxidant activity genes. The generation of intracellular radicals was evaluated using the DCF-DA method in six of the articles that carried out this test, another of the most common analyzes was lipid peroxidation in five of the twelve articles, four of which used the DPPP method. (1,3-bis (diphenylphosphino) propane) and only one reaction with TBARS (Thiobarbituric acid reactive substances). Issac et al.(26) was the one that carried out more exhaustive tests to analyze the presence of enzymes that provide antioxidant protection, these were for superoxide dismutase, catalase, reduced glutathione, glutathione peroxidase, glutathione-Stransferase, and acetylcholinesterase. Rajasekar et al.(28) observed the growth, reproduction, and effect on the tissues, being a longer study than the others, reaching up to 60 d.p.f due to the doses administered in the food.
Chemical compounds
Research is not always about finding new compounds, existing ones can also be given a new use, generally, this is analyzed by observing possible side effects in treatments or by the properties of the compound, it is inferred that they have antioxidant and anti-inflammatory, so they are checked.
These compounds were purchased from different chemical houses for their use, as there is greater diversity in their structure and composition, thus obtaining a greater margin in analysis, times, and conditions.
Certain compounds required analysis of their composition or purity, HPLC was used in the works and no other general method was used to analyze factors that infer the antioxidant activity, being one of the few treatments that stood out that of Rangasamy et al.(41), which measured the concentration of ketoprofen in the water of the fish after the inoculation of the compound.
The times at which the compounds were applied were different zebrafish, those with the lowest application were ketoprofen and resveratrol with 3 h.p.f, followed by lysozyme with 48 h.p.f, between 5 to 7 d.p.f with abamectin, polyethylene terephthalate, fluralan, and MeO- PEG-b-PMOT (methoxy-poly(ethylene glycol)-b-poly(4-[2,2,6,6-tetramethylpiperidine-1-oxyl]oxymethylstyrene), while in the trials where the fish were older it was applied ulexite with 14 d.p.f and vitamin E with 15 d.p.f because it was supplied in the fish feed.
The agent to induce stress was AAPH and hydrogen peroxide, only Carrillo et al.(42); Vong et al.(43) while in the other studies the use of oxidizing agents was not reported.
Only Carrillo et al.(42); Vong et al.(43), Boeri et al.(14); Vong et al.(43) reported mortality and dysmorphology by microscope observation, apoptosis was reported in Ren et al.(44) using acridine orange, and Jiao et al.(45) using the Jiancheng Nanjing Kit.
Alak et al.(46); Boeri et al.(14); dos Santos et al.(47) used the TBARS method for oxidative peroxidation, while Rangasamy et al.(41) used plate fixation and microscope observation for this fin.
The expression of genes related to antioxidant activity in fish was evaluated by real-time PCR, the genes searched for were cyp1a, vtg, β-actin, sod, cat, gpx, Nrf2, ho-1, nt10b, GSK-3β, PPARγ, β-catenin, gapdh, and elfa, these analyzes were performed in five of the studies.
Kits from Nanjing Jiancheng were discovered to be used to assess the activity of enzymes related to the antioxidant capacity of fish, based on the following principles:
· Catalase: spectroscopy of hydrogen peroxide consumption
· Superoxide dismutase: formation of adrenochrome by spectroscopy
· Glutathione peroxidase: NADPH (nicotinamide adenine dinucleotide phosphate) oxidation spectroscopy s-transferase glutathione: spectroscopy of glutathione reduction by CDNB (2,4- dinitrochlorobenzene)
These analyzes were performed on eight of the articles in this section, in addition to other specific ones.
Other sources
Although the main sources are vegetables and their derivatives, novel techniques are chosen to achieve antioxidant effects, one of which is a genetic modification to express genes that promote defense against stressful agents, such as work Sant et al.(48) that inserted the Nrf2a gene and later its expression was analyzed by PCR, mortality with observation under a microscope and apoptosis with the acridine orange method, in vitro analysis such as HPLC was also performed.
Animal sources are not known to produce antioxidants or anti-inflammatories, but certain enzymes can help these purposes, as in the work with chicken egg lysozyme carried out by Carrillo et al.(42), where this enzyme is isolated and provided to the zebrafish. at 48 h.p.f, being an unprecedented experience, several concentrations were carried out to identify the behavior with this compound, in addition, HPLC and mass spectroscopy was carried out for the composition of the administered substance, observation under the microscope for dysmorphogenesis and mortality, while the TBARS method for lipid peroxidation in fish, these methods being those that have been seen in other experiences.
In addition to antioxidant properties, metabolites can provide other benefits, being versatile in their implementation, such is the case of the freeze-dried probiotics of Lactobacillus rhamnosus and Bifidobacterium longum, which were supplied in food for 12 days, as found in other experiences with the same dosage form, the exposure times are high, the behavior was demonstrated by photographs and its mobility, Valcarce et al.(49) emphasized the quality of sperm for its subsequent use in reproduction, indicating how multifaceted the sperm is metabolites.
CONCLUSION
Zebrafish is an excellent model for the study of different kinds of metabolites, as it is difficult to use it for preliminary analyzes of extracts, or already isolated natural and chemical compounds, even genetic engineering is used. The potential of this model is seen in the antioxidant and antiinflammatory effect that can be easily induced using inducing agents, thanks to its rapid growth and possibility to study in early stages of development (from 1 h.p.f), in addition to its physical characteristics such as its transparent skin., which allows us to observe the interior of these animals through microscopy and thus observe the effects on their organs and development. The similarity of the genome with humans has allowed several genes related to the antioxidant and anti-inflammatory capacity to be analyzed by PCR, in addition to the expression of these genes by Western blot, to complement the in vivo studies, several chemical and characterization analyzes are developed. , such as HPLC, DPPH, mass spectroscopy, absorbance, etc., you will be able to know the metabolite in its structure and functional groups. The zebrafish model is easy to use and of moderate costs for its implementation, so it does not require large spaces for its study; For tissue analysis, euthanasia is performed under anesthesia, and tissues are homogenized. With this, enzymatic assays are carried out without inconvenience, and the tissues can be kept for other analyses. Zebrafish's adaptability to different metabolites, ease of handling, and closeness to humans in the genome make it an ideal candidate to use as a model for preclinical analyzes of drugs and new metabolites.
REFERENCES
1. Camacho-Escobar MA, Ramos-Ramos DA, Ávila-Serrano NY, Sánchez-Bernal EI, López-Garrido SJ. Las defensas físico-químicas de las plantas y su efecto en la alimentación de los rumiantes. REVISTA TERRA LATINOAMERICANA. 2020 May 18;38(2):443–53.
2. Camacho-Romero OI, Melgarejo-Gómez S, De-la-Rosa-Torres C. Extracción y evaluación de los metabolitos secundarios de extractos etéreos del fruto Syzygium cumini (Jambool). Revista Tecnología en Marcha. 2017 Apr 21;30(1):113.
3. Castro GD. Dependencia de la dosis en los mecanismos de toxicidad y la evaluación de riesgo en toxicología. Acta Bioquimica Clinica Latinoamericana [Internet]. 2013 [cited 2022 Nov 21]; Available from: http://www.scielo.org.ar/scielo.php?pid=S0325-29572013000300010&script=sci_abstract
4. Saeidnia S, Manayi A, Abdollahi M. From in vitro Experiments to in vivo and Clinical Studies; Pros and Cons. Curr Drug Discov Technol. 2016 Jan 22;12(4):218–24.
5. Moctezuma Viera KR. Utilización de animales en la investigación biomédica y médica. Rev Iberoam Bioet. 2020 Feb 27;(12):01–19.
6. Escobedo-Moratilla A, Barba R, Pérez-Urizar J. Modelos preclínicos in vitro e in vivo para la evaluación de la actividad biológica en estudios de biocomparabilidad. Gac Med Mex [Internet]. 2015 [cited 2022 Nov 26];151:376–86. Available from: https://www.anmm.org.mx/GMM/2015/n3/GMM_151_2015_3_377-386.pdf
7. Acevedo Fernández JJ, Angeles Chimal JS, Rivera HM, Petricevich López VL, Nolasco Quintana NY, Collí Magaña DY, et al. Modelos in vitro para la evaluación y caracterización de péptidos bioactivos. In: Bioactividad de péptidos derivados de proteínas alimentarias. OmniaScience; 2013. p. 29–82.
8. Fina BL, Lombarte M, Rigalli A. Research a natural phenomenon: Studies in vivo, in vitro or in silico? | Investigación de un fenómeno natural: Estudios in vivo, in vitro o in silico? Actualizaciones En Osteologia [Internet]. 2013 [cited 2022 Nov 26];9(3):294–9. Available from: http://www.osteologia.org.ar/files/pdf/rid34_Fina.pdf
9. Lawrence C. The husbandry of zebrafish (Danio rerio): A review. Aquaculture. 2007 Sep;269(1–4):1–20.
10. White RM, Sessa A, Burke C, Bowman T, LeBlanc J, Ceol C, et al. Transparent Adult Zebrafish as a Tool for In Vivo Transplantation Analysis. Cell Stem Cell. 2008 Feb;2(2):183–9.
11. Espinosa MB. The Zebrafish : A Tool in Education Resumen Introducción. Revista de Educación En Biología. 2016;19:11–8.
12. Kettleborough RNW, Busch-Nentwich EM, Harvey SA, Dooley CM, de Bruijn E, van Eeden F, et al. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature. 2013 Apr 17;496(7446):494–7.
13. Arteaga C, Bustillos A, Gómez-Catalán J. Migración de neutrófilos en larvas de pez cebra expuestos a extractos de sofrito de tomate. Arch Latinoam Nutr. 2020 Sep 1;70(3):182–90.
14. Boeri P, Piñuel L, Dalzotto D, Monasterio R, Fontana A, Sharry S, et al. Argentine Patagonia barberry chemical composition and evaluation of its antioxidant capacity. J Food Biochem. 2020 Jul 28;44(7).
15. Cholan PM, Han A, Woodie BR, Watchon M, Kurz AR, Laird AS, et al. Conserved anti-inflammatory effects and sensing of butyrate in zebrafish. Gut Microbes. 2020 Nov 9;12(1):1824563.
16. Perumal S, Gopal Samy M v., Subramanian D. Developmental toxicity, antioxidant, and marker enzyme assessment of swertiamarin in zebrafish ( Danio rerio ). J Biochem Mol Toxicol. 2021 Sep 12;35(9).
17. Poornima S, Nagarjun N, Ponmurugan P, Gnanamangai BM, Narasimman S. Toxicity and antiinflammatory study of Parmotrema austrosinense extract against oxozalone induced intestinal inflammation in zebrafish (Danio rerio) model. Biocatal Agric Biotechnol. 2019 Sep;21:101278.
18. Pradeep PS, Manisha S, Monica Amala Nayaki J, Sivaraman D, Selvaraj R, Seeni S. Potential antioxidant and anti-inflammatory action of Hypericum hookerianum extracts in a liposome system evaluated with zebrafish embryos. J Microencapsul. 2019 Aug 19;1–10.
19. Mohamad Shariff NFS, Singgampalam T, Ng CH, Kue CS. Antioxidant activity and zebrafish teratogenicity of hydroalcoholic Moringa oleifera L. leaf extracts. British Food Journal. 2020 Aug 11;122(10):3129–37.
20. Udaya S, Babu N, Nanjappa DP, Kalladka K, Chakraborty G, Chakraborty A. Evaluation of Toxicity and Antioxidant Property of Cassia fistula Stem Bark Extracts in Zebrafish. Journal of Health and Allied Sciences NU. 2020 Dec 20;10(03):109–15.
21. Wang W, Liu J. Efficient extraction, antioxidant activities and anti-inflammation of polysaccharides from Notopterygium franchetii Boiss. Carbohydr Polym. 2020 Nov;248:116783.
22. Arteaga C, Boix N, Teixido E, Marizande F, Cadena S, Bustillos A. The Zebrafish Embryo as a Model to Test Protective Effects of Food Antioxidant Compounds. Molecules. 2021 Sep.
23. Cho SH, Heo SJ, Yang HW, Ko EY, Jung MS, Cha SH, et al. Protective Effect of 3-Bromo-4,5-Dihydroxybenzaldehyde from Polysiphonia morrowii Harvey against Hydrogen Peroxide-Induced Oxidative Stress In Vitro and In Vivo. J Microbiol Biotechnol. 2019 Aug 28;29(8):1193–203.
24. Endo Y, Muraki K, Fuse Y, Kobayashi M. Evaluation of Antioxidant Activity of Spice-Derived Phytochemicals Using Zebrafish. Int J Mol Sci. 2020 Feb 7;21(3):1109.
25. Kang MC, Kim KN, Kang SM, Yang X, Kim EA, Song CB, et al. Protective effect of dieckol isolated from Ecklonia cava against ethanol caused damage in vitro and in zebrafish model. Environ Toxicol Pharmacol. 2013 Nov;36(3):1217–26.
26. Issac PK, Guru A, Velayutham M, Pachaiappan R, Arasu MV, Al-Dhabi NA, et al. Oxidative stress induced antioxidant and neurotoxicity demonstrated in vivo zebrafish embryo or larval model and their normalization due to morin showing therapeutic implications. Life Sci. 2021 Oct;283:119864.
27. Roberto VP, Surget G, le Lann K, Mira S, Tarasco M, Guérard F, et al. Antioxidant, Mineralogenic and Osteogenic Activities of Spartina alterniflora and Salicornia fragilis Extracts Rich in Polyphenols. Front Nutr. 2021 Aug 18;8.
28. Rajasekar P, Palanisamy S, Anjali R, Vinosha M, Elakkiya M, Marudhupandi T, et al. Isolation and structural characterization of sulfated polysaccharide from Spirulina platensis and its bioactive potential: In vitro antioxidant, antibacterial activity and Zebrafish growth and reproductive performance. Int J Biol Macromol. 2019 Dec;141:809–21.
29. Kim HS, Wang L, Fernando IPS, Je JG, Ko SC, Kang MC, et al. Antioxidant efficacy of (−)-loliolide isolated from Sargassum horneri against AAPH-induced oxidative damage in Vero cells and zebrafish models in vivo. J Appl Phycol. 2020 Oct 25;32(5):3341–8.
30. Xia G, Li X, Zhang Z, Jiang Y. Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce. Open Life Sci. 2021 Jan 29;16(1):92–101.
31. Nguyen TH, Le HD, Nguyen Thi Kim T, Pham The H, Nguyen TM, Cornet V, et al. Anti–Inflammatory and Antioxidant Properties of the Ethanol Extract of Clerodendrum Cyrtophyllum Turcz in Copper Sulfate-Induced Inflammation in Zebrafish. Antioxidants. 2020 Feb 25;9(3):192.
32. Pradeep PS, Manisha S, Monica Amala Nayaki J, Sivaraman D, Selvaraj R, Seeni S. Potential antioxidant and anti-inflammatory action of Hypericum hookerianum extracts in a liposome system evaluated with zebrafish embryos. J Microencapsul. 2019 Aug 19;1–10.
33. Arteaga C, Boix N, Teixido E, Marizande F, Cadena S, Bustillos A. The Zebrafish Embryo as a Model to Test Protective Effects of Food Antioxidant Compounds. Molecules. 2021 Sep 24;26(19):5786.
34. Kim HS, Wang L, Fernando IPS, Je JG, Ko SC, Kang MC, et al. Antioxidant efficacy of (−)-loliolide isolated from Sargassum horneri against AAPH-induced oxidative damage in Vero cells and zebrafish models in vivo. J Appl Phycol. 2020 Oct 25;32(5):3341–8.
35. Xia G, Li X, Zhang Z, Jiang Y. Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce. Open Life Sci. 2021 Jan 29;16(1):92–101.
36. Roberto VP, Surget G, le Lann K, Mira S, Tarasco M, Guérard F, et al. Antioxidant, Mineralogenic and Osteogenic Activities of Spartina alterniflora and Salicornia fragilis Extracts Rich in Polyphenols. Front Nutr. 2021 Aug 18;8.
37. Jayawardena TU, Wang L, Sanjeewa KKA, Kang SI, Lee JS, Jeon YJ. Antioxidant Potential of Sulfated Polysaccharides from Padina boryana; Protective Effect against Oxidative Stress in In Vitro and In Vivo Zebrafish Model. Mar Drugs. 2020 Apr 14;18(4):212.
38. Cho SH, Heo SJ, Yang HW, Ko EY, Jung MS, Cha SH, et al. Protective Effect of 3-Bromo-4,5-Dihydroxybenzaldehyde from Polysiphonia morrowii Harvey against Hydrogen Peroxide-InducedOxidative Stress In Vitro and In Vivo. J Microbiol Biotechnol. 2019 Aug 28;29(8):1193–203.
39. Kim HS, Wang L, Fernando IPS, Je JG, Ko SC, Kang MC, et al. Antioxidant efficacy of (−)-loliolide isolated from Sargassum horneri against AAPH-induced oxidative damage in Vero cells and zebrafish models in vivo. J Appl Phycol. 2020 Oct 25;32(5):3341–8.
40. Endo Y, Muraki K, Fuse Y, Kobayashi M. Evaluation of Antioxidant Activity of Spice-Derived Phytochemicals Using Zebrafish. Int J Mol Sci. 2020 Feb 7;21(3):1109.
41. Rangasamy B, Hemalatha D, Shobana C, Nataraj B, Ramesh M. Developmental toxicity and biological responses of zebrafish (Danio rerio) exposed to anti-inflammatory drug ketoprofen. Chemosphere. 2018 Dec;213:423–33.
42. Carrillo W, Gómez-Ruiz JA, Miralles B, Ramos M, Barrio D, Recio I. Identification of antioxidant peptides of hen egg-white lysozyme and evaluation of inhibition of lipid peroxidation and cytotoxicity in the Zebrafish model. European Food Research and Technology. 2016 Oct 1;242(10):1777–85.
43. Vong LB, Kobayashi M, Nagasaki Y. Evaluation of the Toxicity and Antioxidant Activity of Redox Nanoparticles in Zebrafish ( Danio rerio ) Embryos. Mol Pharm. 2016 Sep 6;13(9):3091–7.
44. Ren F, Huang Y, Tao Y, Ji C, Aniagu S, Jiang Y, et al. Resveratrol protects against PM2.5-induced heart defects in zebrafish embryos as an antioxidant rather than as an AHR antagonist. Toxicol Appl Pharmacol. 2020 Jul;398:115029.
45. Jiao Y, Tao Y, Yang Y, Diogene T, Yu H, He Z, et al. Monobutyl phthalate (MBP) can dysregulate the antioxidant system and induce apoptosis of zebrafish liver. Environmental Pollution. 2020 Feb;257:113517.
46. Alak G, Ucar A, Parlak V, Yeltekin AÇ, Özgeriş FB, Atamanalp M, et al. Antioxidant Potential of Ulexite in Zebrafish Brain: Assessment of Oxidative DNA Damage, Apoptosis, and Response of Antioxidant Defense System. Biol Trace Elem Res. 2021 Mar 15;199(3):1092–9.
47. dos Santos MM, de Macedo GT, Prestes AS, Ecker A, Müller TE, Leitemperger J, et al. Modulation of redox and insulin signaling underlie the anti-hyperglycemic and antioxidant effects of diphenyl diselenide in zebrafish. Free Radic Biol Med. 2020 Oct;158:20–31.
48. Sant KE, Sinno PP, Jacobs HM, Timme-Laragy AR. Nrf2a modulates the embryonic antioxidant response to perfluorooctanesulfonic acid (PFOS) in the zebrafish, Danio rerio. Aquatic Toxicology. 2018 May;198:92– 102.
49. Valcarce DG, Riesco MF, Martínez-Vázquez JM, Robles V. Diet Supplemented with Antioxidant and AntiInflammatory Probiotics Improves Sperm Quality after Only One Spermatogenic Cycle in Zebrafish Model. Nutrients. 2019 Apr 13;11(4):843.
FINANCIACIÓN
Los autores agradecemos a la Dirección de Investigación y Desarrollo DIDE por el financiamiento, a través del proyecto titulado: “Evaluación de la actividad antimicrobiana y antibiofilm de aceites esenciales micro encapsulados”.
CONFLICTO DE INTERESES
Los autores declaran que no existe conflicto de intereses
CONTRIBUCIÓN DE AUTORÍA
Conceptualización: Alberto Bustillos.
Investigación: Alberto Bustillos, Cristina Arteaga, Ruth Borja.
Metodología: Alberto Bustillos, Luis Felipe Contreras, Carolina Campoverde.
Redacción – borrador original: Alberto Bustillos, Cristina Arteaga, Ruth Borja, Luis Felipe Contreras, Carolina Campoverde.
Redacción – revisión y edición: Alberto Bustillos, Cristina Arteaga, Ruth Borja, Luis Felipe Contreras, Carolina Campoverde.